SEM Haplotype characterization POC_ID_relative abundace project
SEM Haplotype characterization | POC_ID
Summary
As part of my PhD project on Settlement and post settlement challenges to successful recruitment for reef building corals the second chapter focused on the relative abundance of Pocillopora sp. within the Moorea lagoon. A characterization study of the calcareous skeleton by Haplotype (Johnston et al 2021) will be carried out. In addition to genetic identification, this study will enable us to understand the plasticity of each Haplotype as a function of its natural environment. It will also highlight the great interspecific diversity of Pocillopora sp. populations. Analysis of the fragments will be carried out at Haifa University using the SEM 3D Imagine technique (Goodbody-Gringley & Waletich, 2018; Doherty, Matthew et al. 2023)
Protocol
Data
The samples used in this process come from the relative abundance project transects (POC-ID). The complete data are located on a table constituting the tracking of each of them. This can be found at the following link. For each modification or fragmentation, the columns Image-Sample, Bag-number, Storage, Image-sample-backup, Bag-number-backup, Storage_backup must be completed and updated as frequently as possible. Each fragment is stored in Whirlpak bags and stored in freezers at -40°C within a meshbag numbered by Site, Transect and Date. For each sample (Whirlpak), number sample, project ID POC-ID and name of the leader of project PH are recorded.
Fragmentation
The aim of the manipulation is to have a fragment with a size of about 1cmX1cm in order to have a sufficiently large analysis surface to facilitate SEM imaging at Haifa University. A backup fragment will also be created to keep a fragment at the GUMP Field Station in case of any problems. Corals will be cut using the Gryphon diamond band saw (cutting protocol can be found on the following link): link
Materials
- Coral saw (Gryphon diamond band saw C-140)
- Plastic cup
- Zip block bag size 2 “X3” (2ml)
- Stickers (1 for sample- 1 for backup smaple) with sample number, date, site, transect on it. Write backup on second zipblock bag
- Waterproof papers (1 for sample- 1 for backup smaple) with sample number, date, site, transect on it. Write backup on second zipblock bag
- Sharpie pencil
- Bleach
- Gloves
- Fresh water
- Paper towel
- Drying plate to dry bleach fragments before putting them in zipblock bag
Sample preparation for shipping
Before cutting the fragments, perform the following steps:
- Number the plastic cup with sample number, site, transect.
- Number the Sticker (2 times) and waterproof paper (2 times) with sample number, date, site, transect. For the second sticker and paper, enter backup.
- Dilute bleach to 20%.
- Cut fragments (sample, sample backup) following fragmentation steps with Gryphon diamond band saw c-140.
- Fill plastic cup with bleach diluted to 20% to cover fragments (sample, sample backup) (10ml should be sufficient)
- Wait 24h processing time with bleach to ensure that no coral tissue remains on the fragment.
- Rinse fragments with fresh water to remove remaining bleach
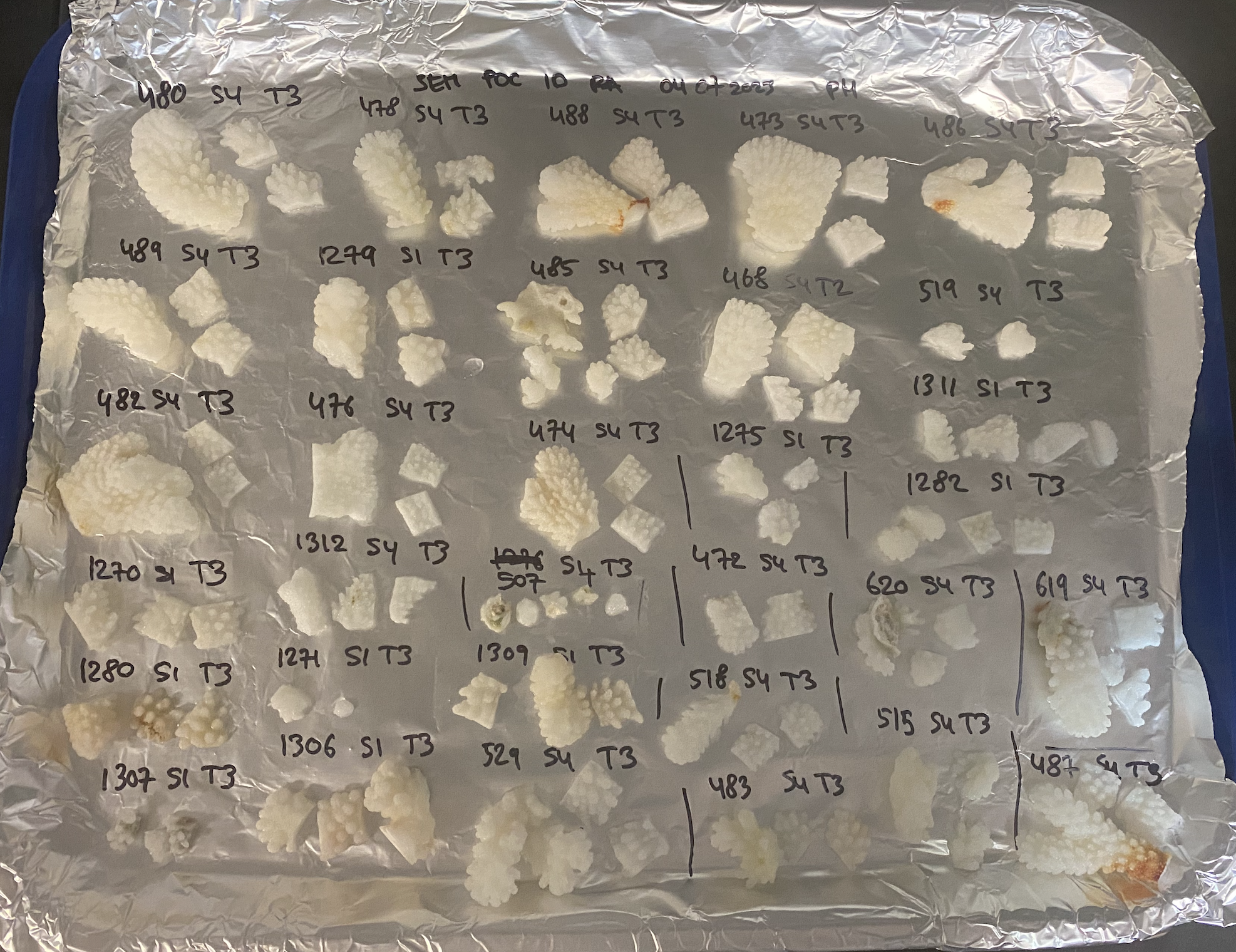
- Place the fragment on an aluminium foil on a tray dedicated to Dryoven
- Put the fragments (sample, sample backup) on the plate, writting on the aluminium feuille sample number, site, transect and place the fragments under the notes to track the samples during the drying period. Perform the drying period over a period of 24h
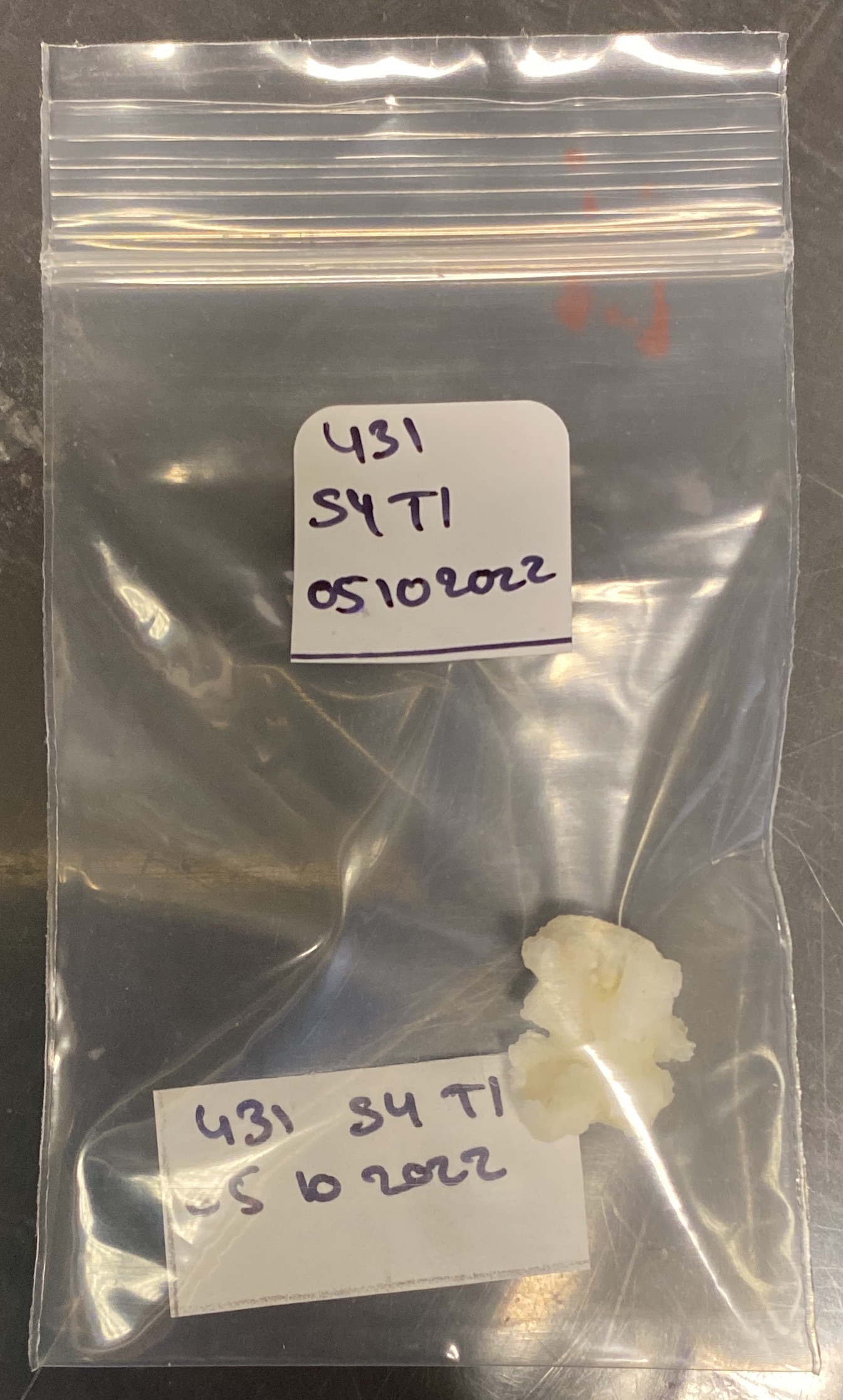
- Stick the stickers from step 2 onto the zipblock bag
- Wrap the sample with a square of paper towel previously cut from the sample to be shipped. Do not wrap a paper towel around the second sample
- Place the papers and fragments in the zipblock bag concerned. The resultat need to be as the pictures below
- Put the zipblock bag of the fragment destined for SEM analysis into the box, respecting the site/transect organization, then do the same for the sample backup remaining at GUMP Field Sation.
- Compile excel spreadsheet on GitHub to track samples
Storage
Corals leaving for SEM analysis will be stored by Site/Transect and then stored in a box containing all samples. Corals remaining in Moorea will be stored by Site/Transect and then stored in a box containing all samples.