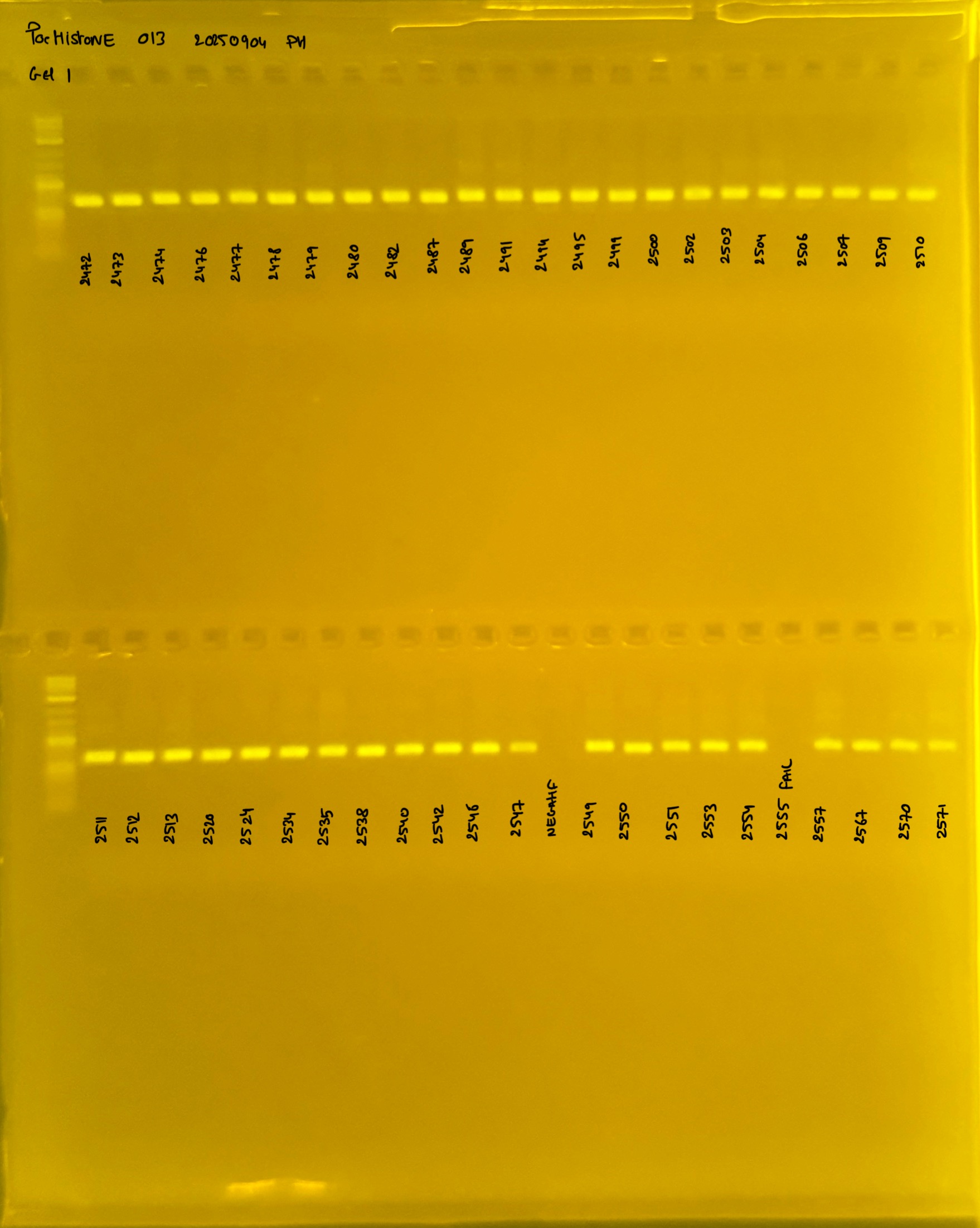
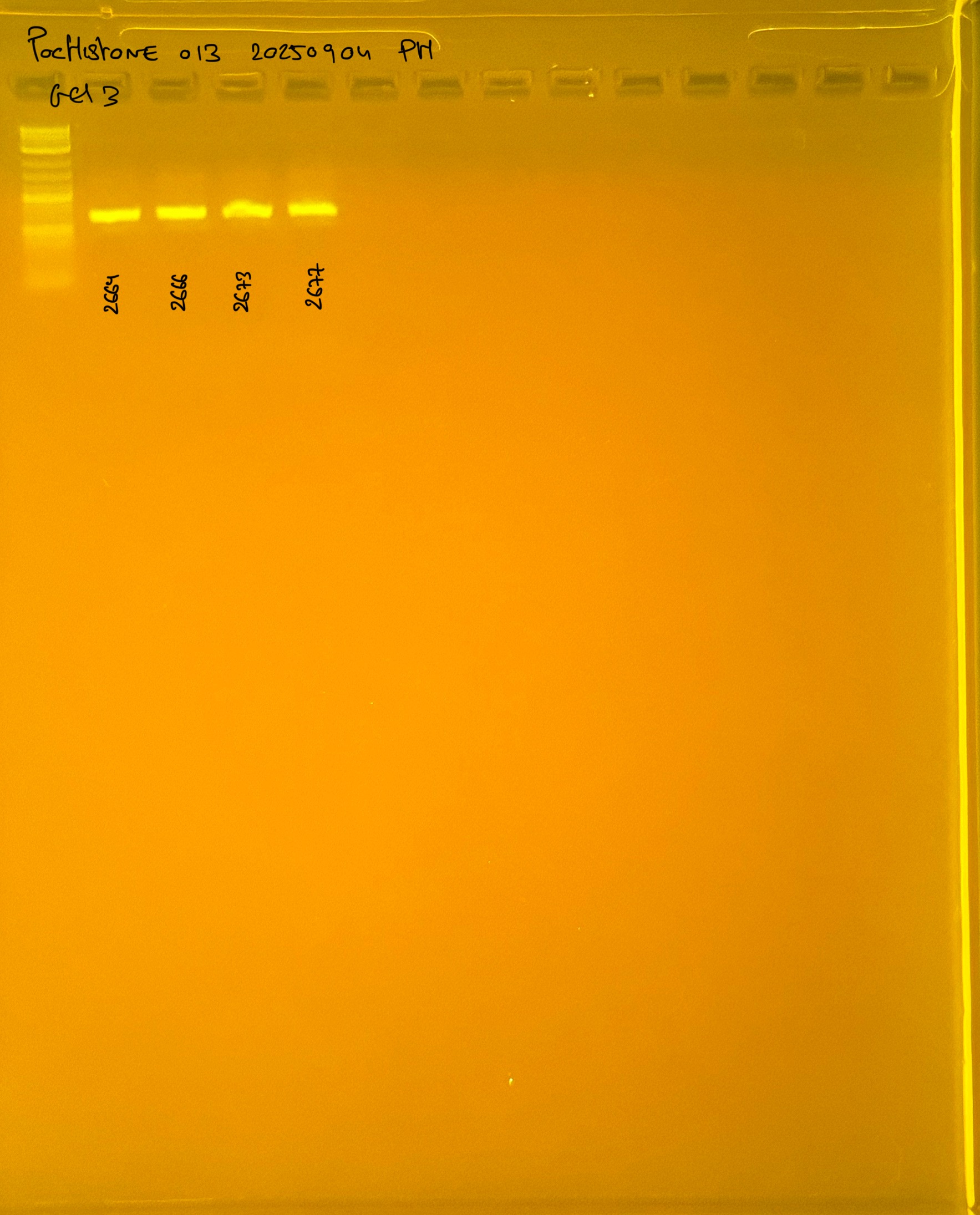
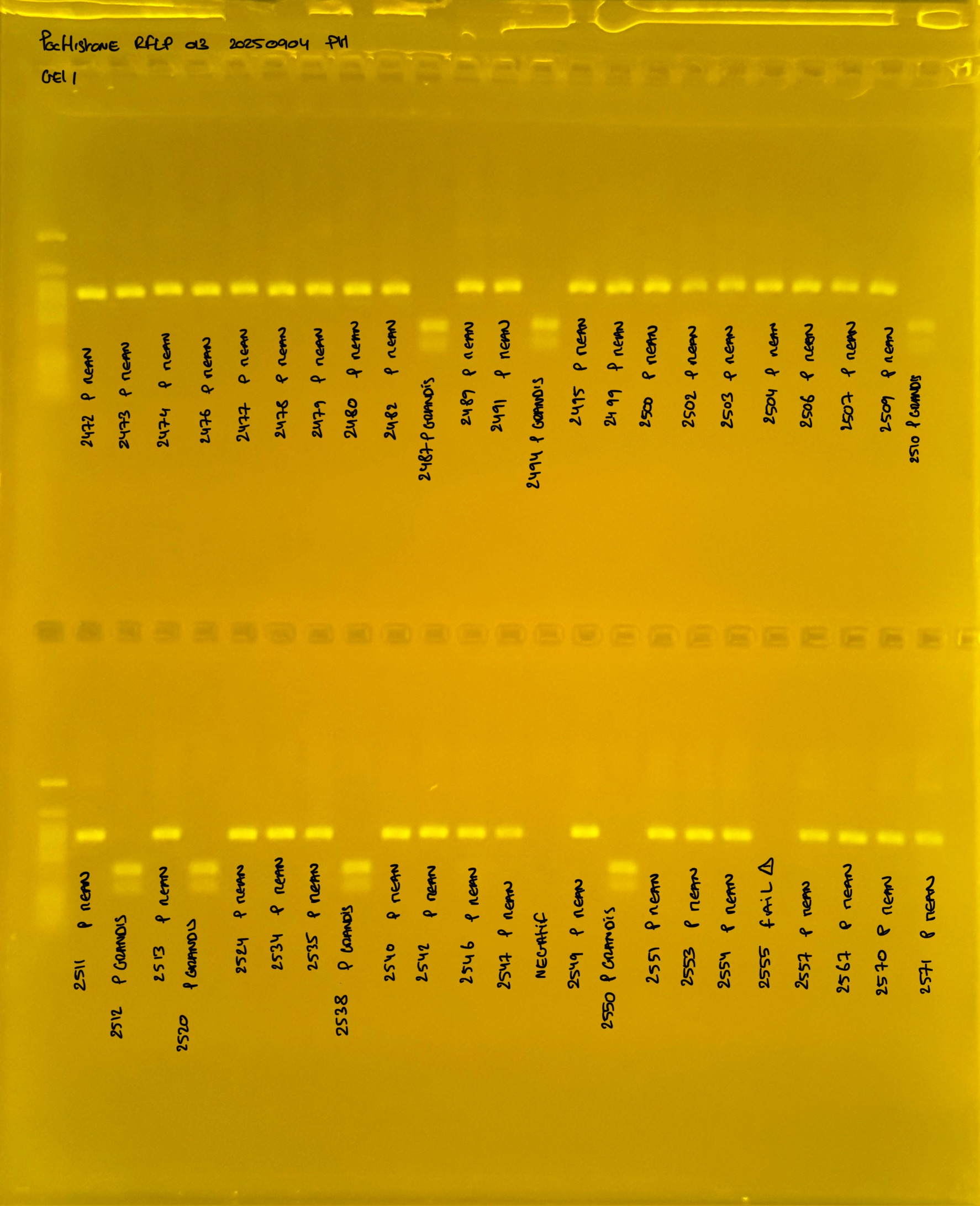
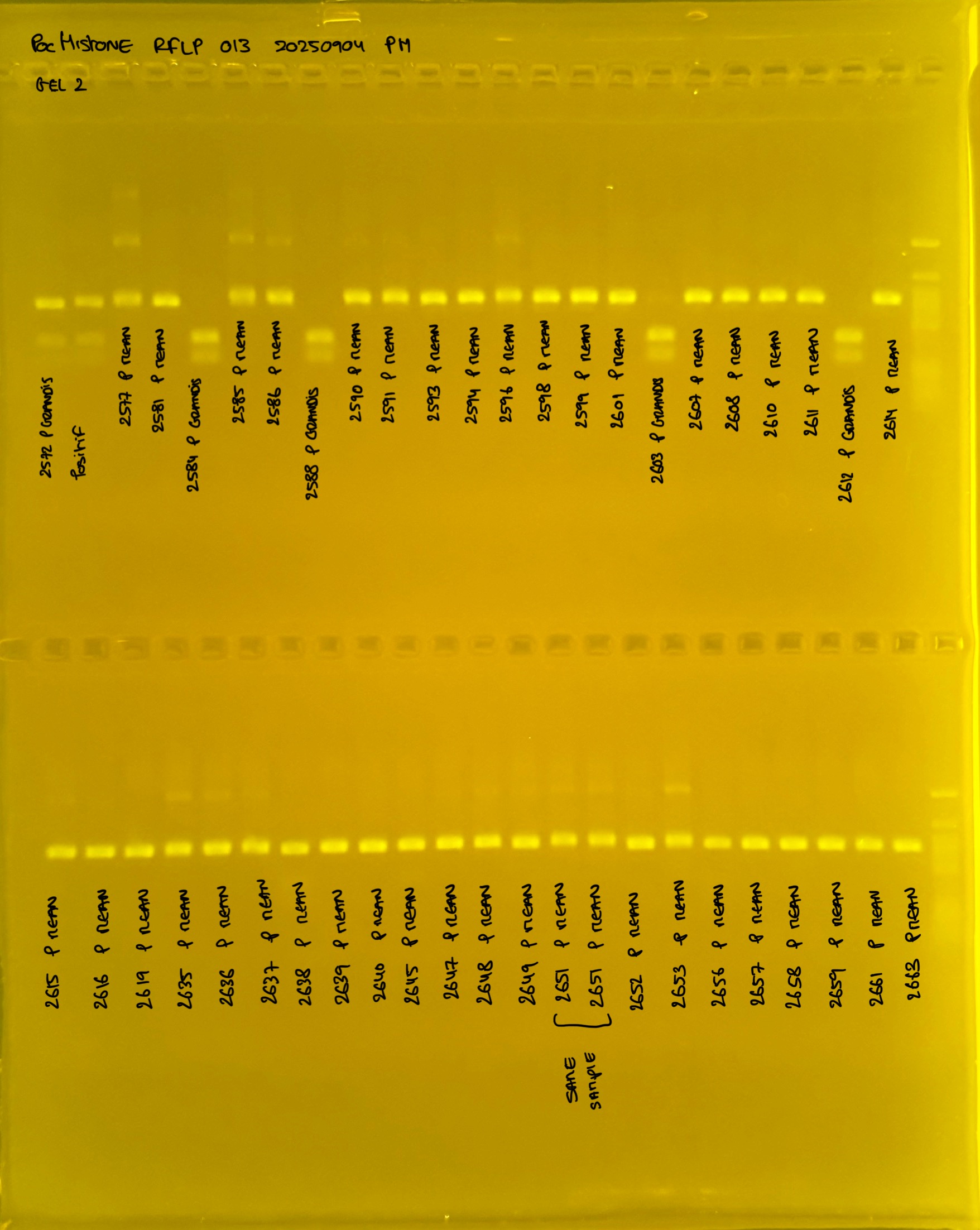
PocID Relative abundance molecular species ID.
Relative abundance Pocillopora spp. around the island of Mo’orea. Species identification via mtORF-PocHistone and RFLP.
Context:
Our study is based on population genetics of Pocillopora sp. on the Polynesian islands based on their often misleading and incorrect morphology. We are therefore trying to understand population dynamics by Haplotype so that we can correlate our transect data with our term tolerance data from the laboratory (see NoteBook TPC Haplotype).
gDNA extraction with “Quick-DNA 96 Kit / Cat No: D3012
Protocol:
-
Reagent preparation: Add 260ul or 1,040ul Proteinase K storage Buffer to reconstitute the lyophilized Proteinase K, 5mg (D3001-2-5) or 20mg (D3001-2-20), respectively (final concentration of 20mg/ml). Vortex to dissolve.
-
gDNA extraction in DNA/RNA shield
-
Add 10ul PK Digestion buffer + 5ul of Proteinase K.
For 2 plate in a time –> 2.0ml of PK Digestion Buffer + 1.0ml ProtK -
Add 400ul og Genomic Lysis Buffer to 100ul of teh sample/shield mixture.
For 2 plate in a time –> 400ul x 192 samples = 76.8ml -
Mix completely by vortexing (with pipette in the plate) 4-5 times then let stand 10 minutes
-
Transfer the mixture to the wells of a Silicon-A Plate on a Collection Plate. Centrifuge at 2.500xg for 5 minutes.
-
Remove the waste in the colelction plate and move to the next step.
-
Add 200ul of DNA Pre-Wash Buffer to each well and. centrifuge at 2,500xg for 5 nminutes,
For 2 plate in a time –> 200ul x 192 samples = 38.4ml. -
Remove the waste in the colelction plate and move to the next step.
-
Add 300ul g-DNA Wash Buffer to each well and centrifuge at 2,500xg for 5 minutes.
For 2 plate in a time –> 300ul x 192 samples = 57.6ml. -
Transfer the Silicon-A Plate onto an Elution plate. Add 50ul DNA Elution Buffer (need to be heat at 70˚C) to each well and incubate during 2 minutes then centrifuge at 2,500xg for 5 minutes. For 2 plate in a time –> 50ul x 192 samples = 9.6ml.
-
Keep the Silicon-A Plate in the fridge (4˚C).
-
Cover the Elution Plate and keep it at -20˚C.
-
Nano drop 3-4 samples on each plate to control de DNA concentration.
-
Nano drop result :
20240919 Plate 16 and 17.
See plate number for each samples here.
| Plate | Sample | Well | ng/ul | A260/280 | A260/230 |
|---|---|---|---|---|---|
| 17 | 1398 | A1 | 17 | 1.72 | 0.85 |
| 17 | 1390 | B1 | 1.1 | 1.6 | 0.16 |
| 16 | 1407 | H12 | 1.5 | 2.75 | 0.56 |
| 16 | 2220 | A1 | .- 0,8 | 0.84 | .-0.06 |
| 16 | 2194 | B1 | .-0.9 | 1.16 | .-0.25 |
After checking we figure it out that we didin’t add Elution Buffer but g-DNA Wash buffer. Following that we tooked plates out of the freezer and added 50ul of Elution Buffer Eluted in 50ul of Elution Buffer and mesured on nanodrop.
| Plate | Sample | Well | ng/ul | A260/280 | A260/230 |
|---|---|---|---|---|---|
| 16 | 2220 | A1 | 3.4 | 1.97 | 0.89 |
| 16 | 2194 | B1 | 20.8 | 1.88 | 1.83 |
| 16 | 2189 | C1 | 13.6 | 1.9 | 1.96 |
| 17 | 1398 | A1 | 10 | 1.82 | 1.01 |
| 17 | 1390 | B1 | 14.9 | 1.86 | 2.32 |
| 17 | 2163 | C1 | 12.1 | 1.91 | 1.31 |
20240920 Plate 18 and 19.
See plate number for each samples here.
| Plate | Sample | Well | ng/ul | A260/280 | A260/230 |
|---|---|---|---|---|---|
| Elution buffer | x | x | 0.1 | .-0.14 | 0.18 |
| 18 | 2418 | A1 | 15.6 | 2.11 | 2.3 |
| 18 | 2350 | B1 | 23.8 | 2.05 | 0.33 |
| 18 | 2421 | C1 | 31.2 | 2.03 | 1.64 |
| 19 | 1448 | A1 | 16.9 | 2.1 | 2.16 |
| 19 | 1442 | B1 | 12 | 2.14 | 2.22 |
| 19 | 1418 | C1 | 4.1 | 2.83 | 0.56 |
20240920 Plate 20 and 21.
See plate number for each samples here.
| Plate | Sample | Well | ng/ul | A260/280 | A260/230 |
|---|---|---|---|---|---|
| Elution buffer | x | x | 0.1 | .-1.64 | 0.58 |
| 20 | 1424 | A1 | 14.3 | 2.08 | 2.05 |
| 20 | 2463 | B1 | 33.2 | 1.97 | 1.46 |
| 20 | 3314 | C1 | 28.1 | 1.94 | 1.58 |
| 21 | 2137 | A1 | 5.6 | 2.11 | 1.04 |
| 21 | 2152 | B1 | 10.4 | 1.97 | 2.22 |
| 21 | 3113 | C1 | 24.4 | 1.97 | 2.02 |
Probability of mixing buble surface between 12C and 12D on plate 20.
20240920 Plate 22 and 23.
See plate number for each samples here.
| Plate | Sample | Well | ng/ul | A260/280 | A260/230 |
|---|---|---|---|---|---|
| Elution buffer | x | x | 0.8 | .-12.88 | 2.26 |
| 22 | 2136 | A1 | 18.1 | 1.94 | 1.48 |
| 22 | 3231 | B1 | 39 | 1.95 | 1.89 |
| 22 | 3291 | C1 | 22.8 | 1.98 | 1.64 |
| 23 | 3496 | A1 | 25.8 | 2 | 2.46 |
| 23 | 3620 | B1 | 19 | 1.98 | 1.65 |
| 23 | 2916 | C1 | 37.6 | 1.93 | 2.42 |
20240921 Plate 24 and 25.
See plate number for each samples here.
| Plate | Sample | Well | ng/ul | A260/280 | A260/230 |
|---|---|---|---|---|---|
| Elution buffer | x | x | 0 | .-0.01 | 0.12 |
| 24 | 3513 | A12 | 61.8 | 1.96 | 2.33 |
| 24 | 3501 | B12 | 34 | 2.02 | 1.75 |
| 24 | 3674 | C12 | 30.8 | 2.01 | 1.76 |
| 25 | 2752 | F12 | 21.8 | 2.05 | 2.45 |
| 25 | 2781 | G12 | 20.1 | 2.07 | 2.26 |
| 25 | 2768 | H12 | 43.4 | 2.01 | 2.27 |
20240922 Plate 26 and 27.
See plate number for each samples here.
| Plate | Sample | Well | ng/ul | A260/280 | A260/230 |
|---|---|---|---|---|---|
| Elution buffer | x | x | 0.2 | 2.78 | 0.43 |
| 26 | 2715 | A1 | 39 | 1.93 | 1.72 |
| 26 | 2708 | B1 | 35.4 | 1.9 | 0.93 |
| 26 | 2845 | C1 | 32 | 1.95 | 1.55 |
| 27 | 3445 | A12 | 42.9 | 1.92 | 0.84 |
| 27 | 3679 | B12 | 8.9 | 2.11 | 1.83 |
| 27 | 2694 | C12 | 15.9 | 1.98 | 1.4 |
20240922 Plate 28 and 29.
See plate number for each samples here.
| Plate | Sample | Well | ng/ul | A260/280 | A260/230 |
|---|---|---|---|---|---|
| Elution buffer | x | x | .-0.4 | 1.3 | .-5.44 |
| 28 | 2386 | F1 | 33.5 | 1.91 | 1.94 |
| 28 | 2382 | G1 | 38 | 1.93 | 1.9 |
| 28 | 2361 | H1 | 35.9 | 1.92 | 1.88 |
| 29 | 2996 | A1 | 34.1 | 1.95 | 1.6 |
| 29 | 2855 | B1 | 42.1 | 1.9 | 1.09 |
| 29 | 2605 | C1 | 35.2 | 1.95 | 2.14 |
20240922 Plate 30 and 31.
See plate number for each samples here.
| Plate | Sample | Well | ng/ul | A260/280 | A260/230 |
|---|---|---|---|---|---|
| Elution buffer | x | x | .-0.1 | 0.27 | .-0.95 |
| 31 | 3385 | A12 | 31.9 | 1.93 | 2.05 |
| 31 | 3364 | B12 | 29.1 | 1.94 | 2.02 |
| 31 | 3431 | C12 | 10.5 | 1.83 | 0.53 |
| 30 | 2665 | F12 | 35.8 | 1.91 | 2.18 |
| 30 | 2991 | G12 | 28.5 | 1.94 | 1.63 |
| 30 | 2500 | H12 | 47.9 | 1.9 | 1.81 |
20240923 Plate 32 and 33.
See plate number for each samples here.
| Plate | Sample | Well | ng/ul | A260/280 | A260/230 |
|---|---|---|---|---|---|
| Elution buffer | x | x | .-0.1 | 0.18 | .-0.18 |
| 32 | 430 | A1 | 35.6 | 1.96 | 2.1 |
| 32 | 2085 | B1 | 12.6 | 2.1 | 1.62 |
| 32 | 1975 | C1 | 36.8 | 1.99 | 1.87 |
| 33 | 2004 | A12 | 34.4 | 1.95 | 1.38 |
| 33 | 1914 | B12 | 29.6 | 2 | 1.71 |
| 33 | 1779 | C12 | 19.9 | 2.04 | 2.08 |
20240923 Plate 34 and 35.
See plate number for each samples here.
| Plate | Sample | Well | ng/ul | A260/280 | A260/230 |
|---|---|---|---|---|---|
| Elution buffer | x | x | 0.2 | .-0.45 | 0.88 |
| 34 | 1530 | A1 | 19.7 | 1.97 | 1.68 |
| 34 | 1659 | B1 | 24.7 | 2.02 | 1.87 |
| 34 | 1567 | C1 | 22.6 | 2.02 | 2.07 |
| 35 | 1527 | F12 | 46.5 | 1.92 | 2.11 |
| 35 | 1928 | G12 | 31.1 | 1.94 | 1.63 |
| 35 | 1985 | H12 | 38.9 | 1.95 | 2.19 |
20240924 Plate 36 and 37.
See plate number for each samples here.
| Plate | Sample | Well | ng/ul | A260/280 | A260/230 |
|---|---|---|---|---|---|
| Elution buffer | x | x | 0.1 | .-0.09 | .-0.32 |
| 36 | 1959 | A1 | 62.1 | 1.93 | 2.37 |
| 36 | 1963 | B1 | 16.5 | 2.03 | 2.28 |
| 36 | 2025 | C1 | 23.5 | 1.99 | 2.64 |
| 37 | 931 | A10 | 54.4 | 1.96 | 2.13 |
| 37 | 1218 | A11 | 32.7 | 1.93 | 2.7 |
| 37 | 1075 | A12 | 47.8 | 1.93 | 1.81 |
20240925 Plate 38 and 39.
See plate number for each samples here.
| Plate | Sample | Well | ng/ul | A260/280 | A260/230 |
|---|---|---|---|---|---|
| Elution buffer | x | x | 0.3 | .-0.30 | .-1.36 |
| 38 | 814 | A1 | 31 | 1.99 | 2.13 |
| 38 | 892 | A2 | 45.1 | 1.97 | 1.8 |
| 38 | 799 | A3 | 24.4 | 2.04 | 1.16 |
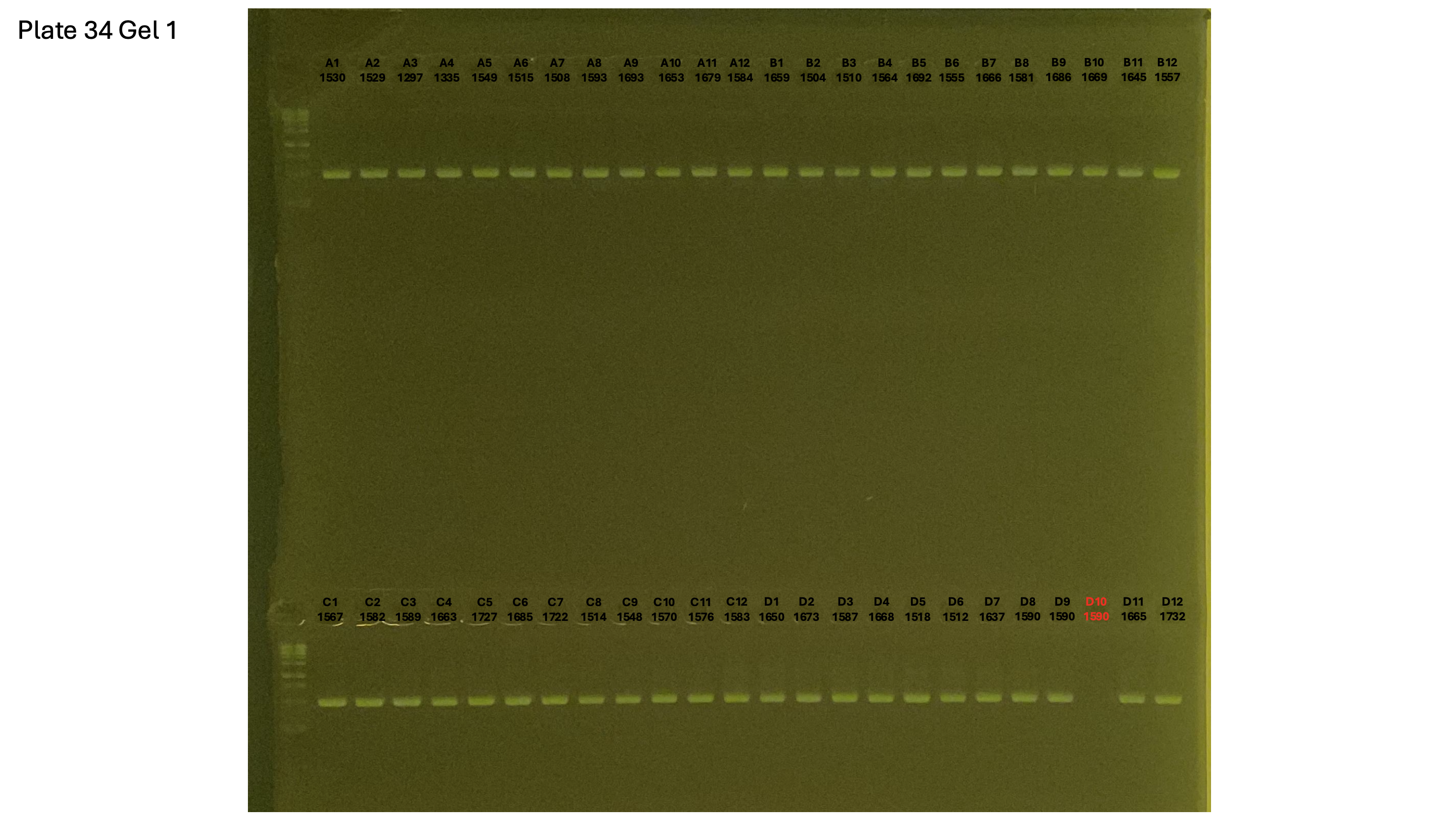
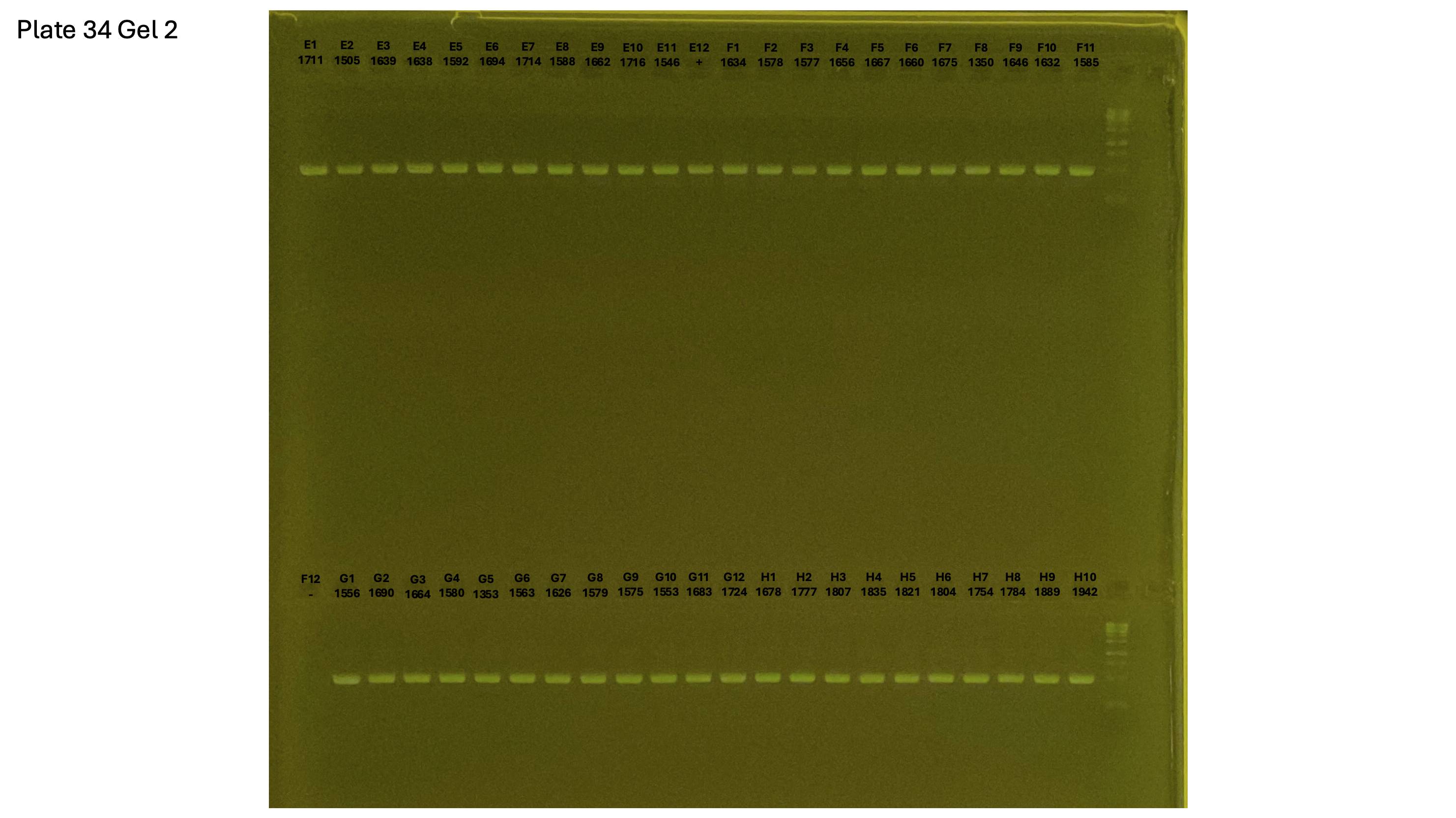
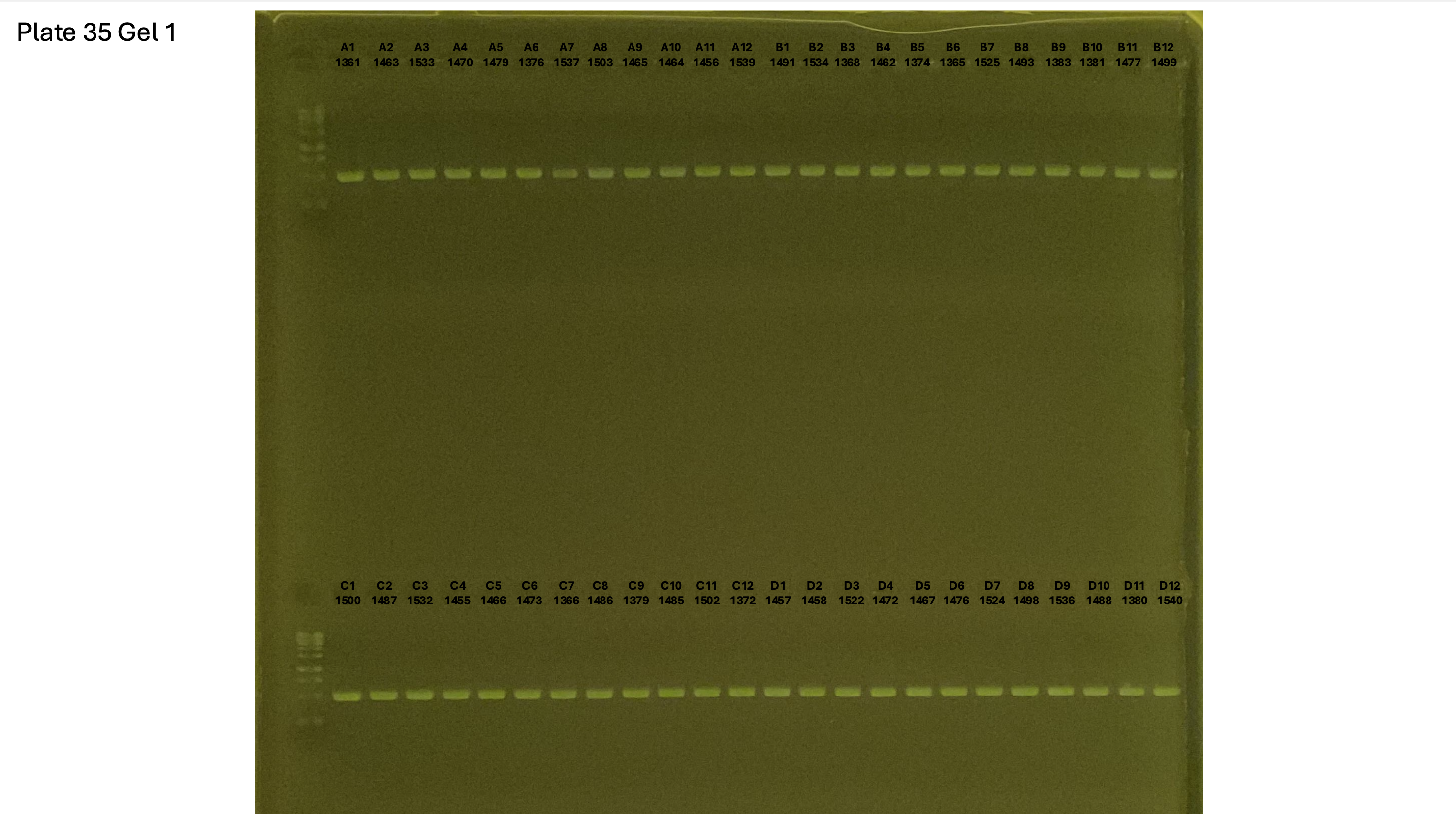
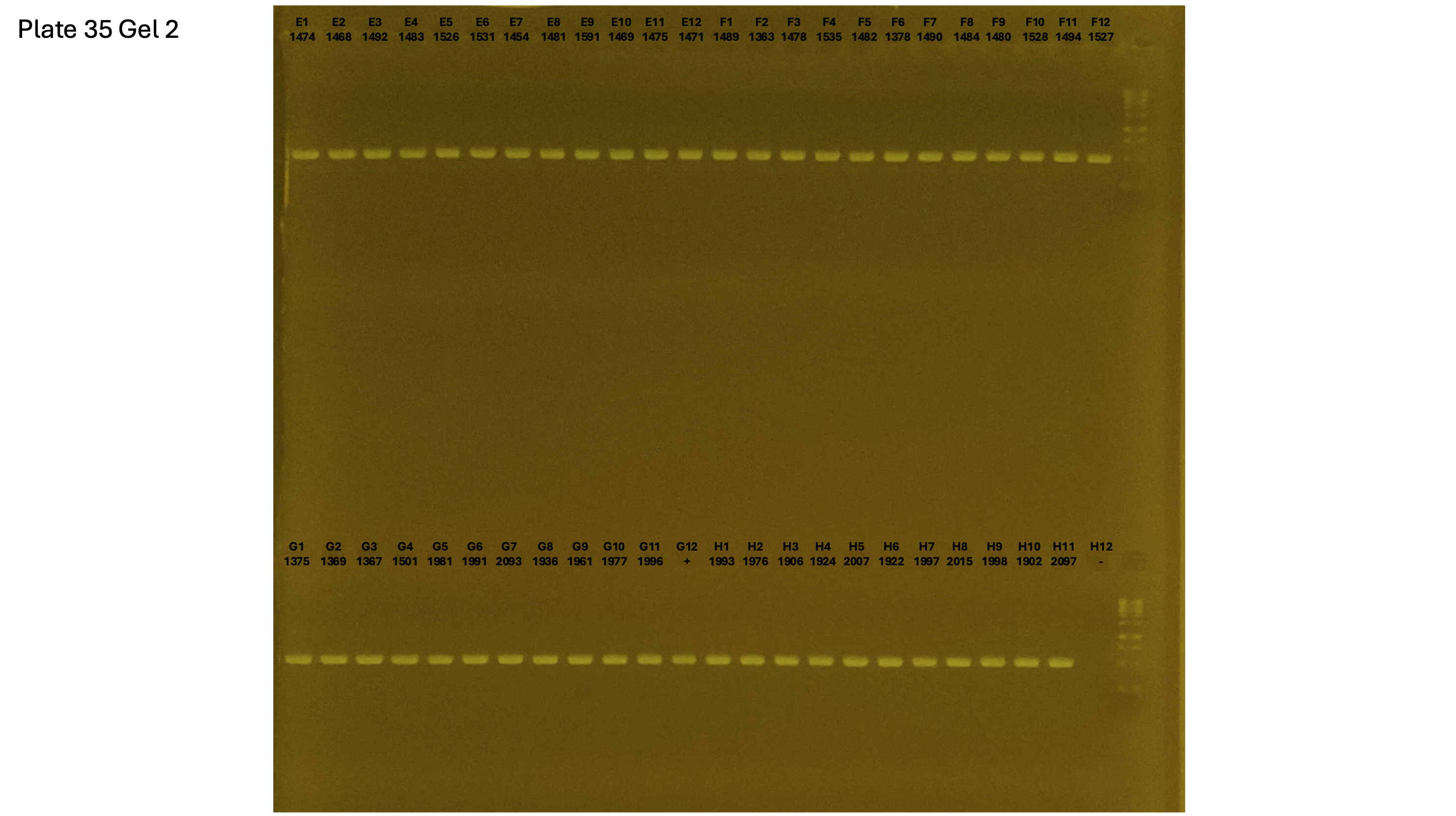
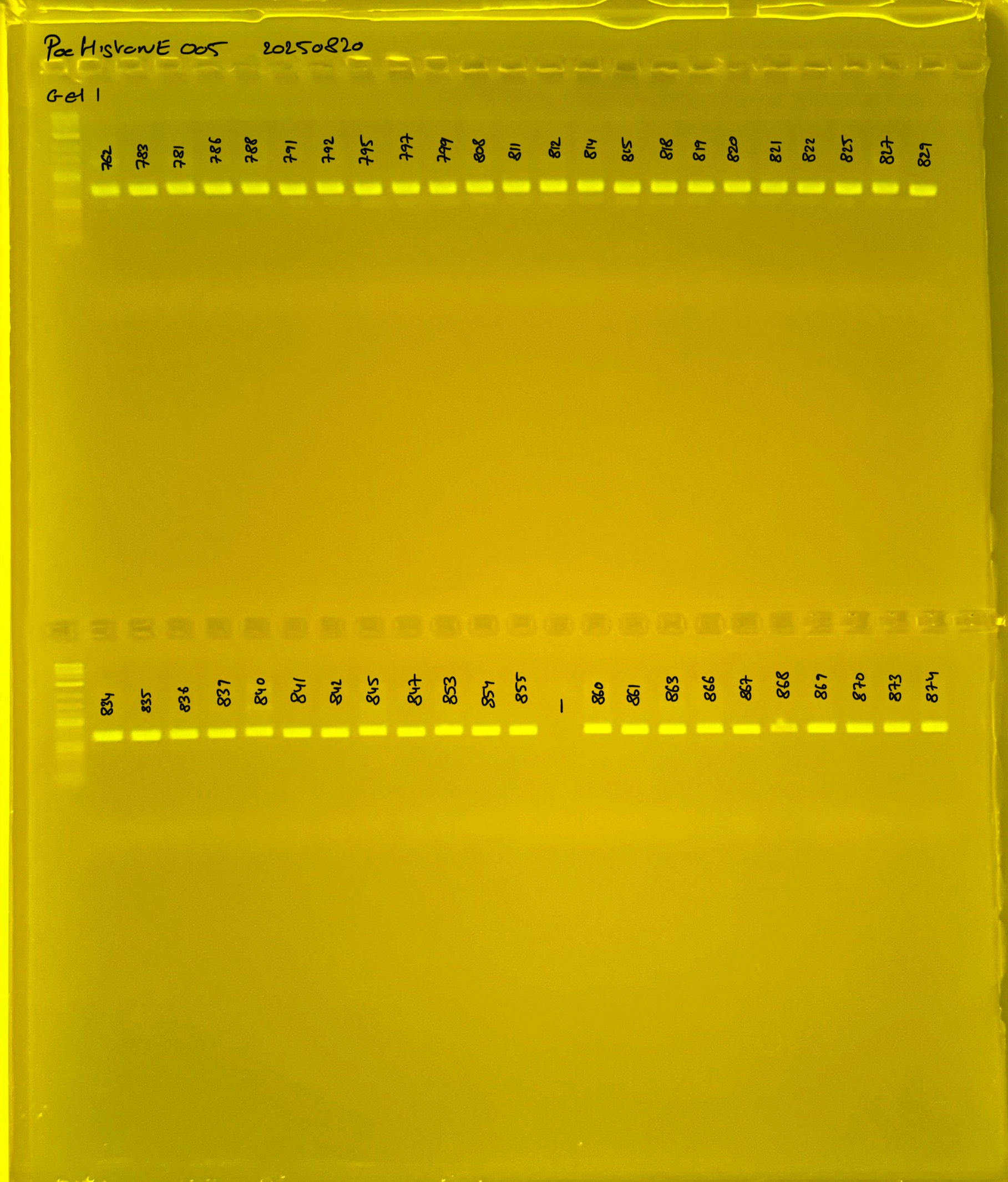
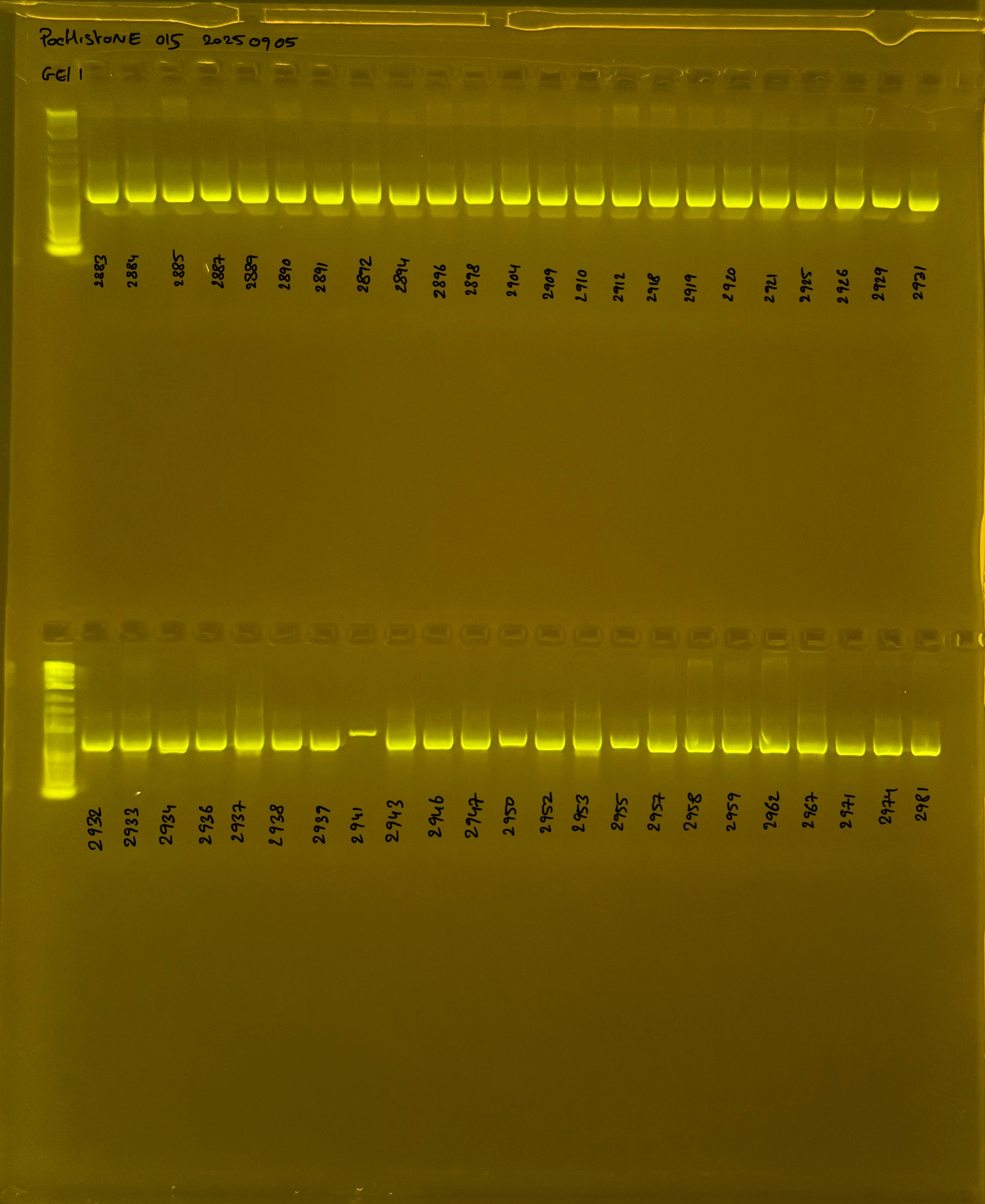
Gel process control of DNA quality
Protocol:
During the process we used differentes size of gel. For this raison we have list bellow all the different way to make the gel at 1.5%.
Small size of gel:
- 1.12g of Agarose
- 75ml of 1x TAE Buffer
- 1µl of gelgreen.
Medium size of gel:
- 1.50g of Agarose
- 100ml of 1x TAE Buffer
- 1µl of gelgreen.
Large size of gel:
- 2.25 of Agarose
- 150ml of 1x TAE Buffer
- 1µl of gelgreen.
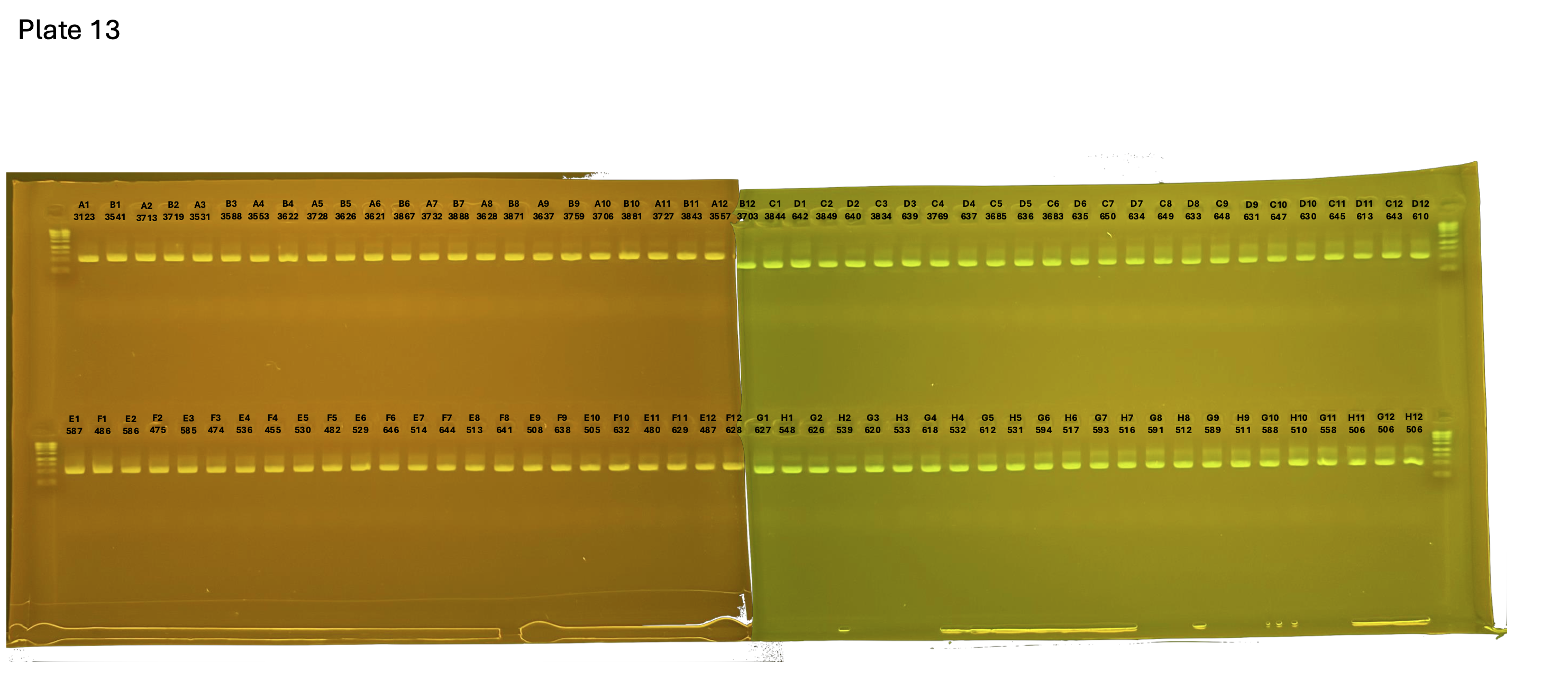
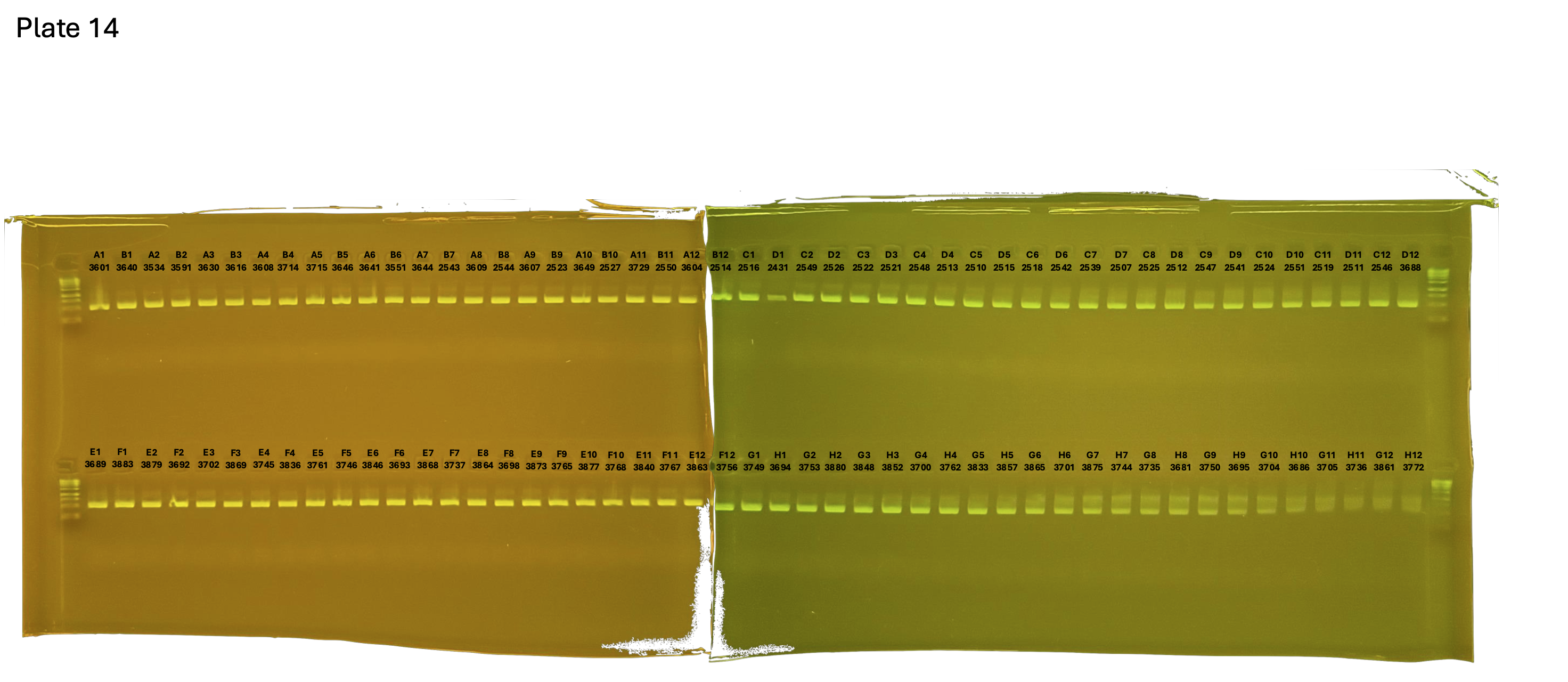
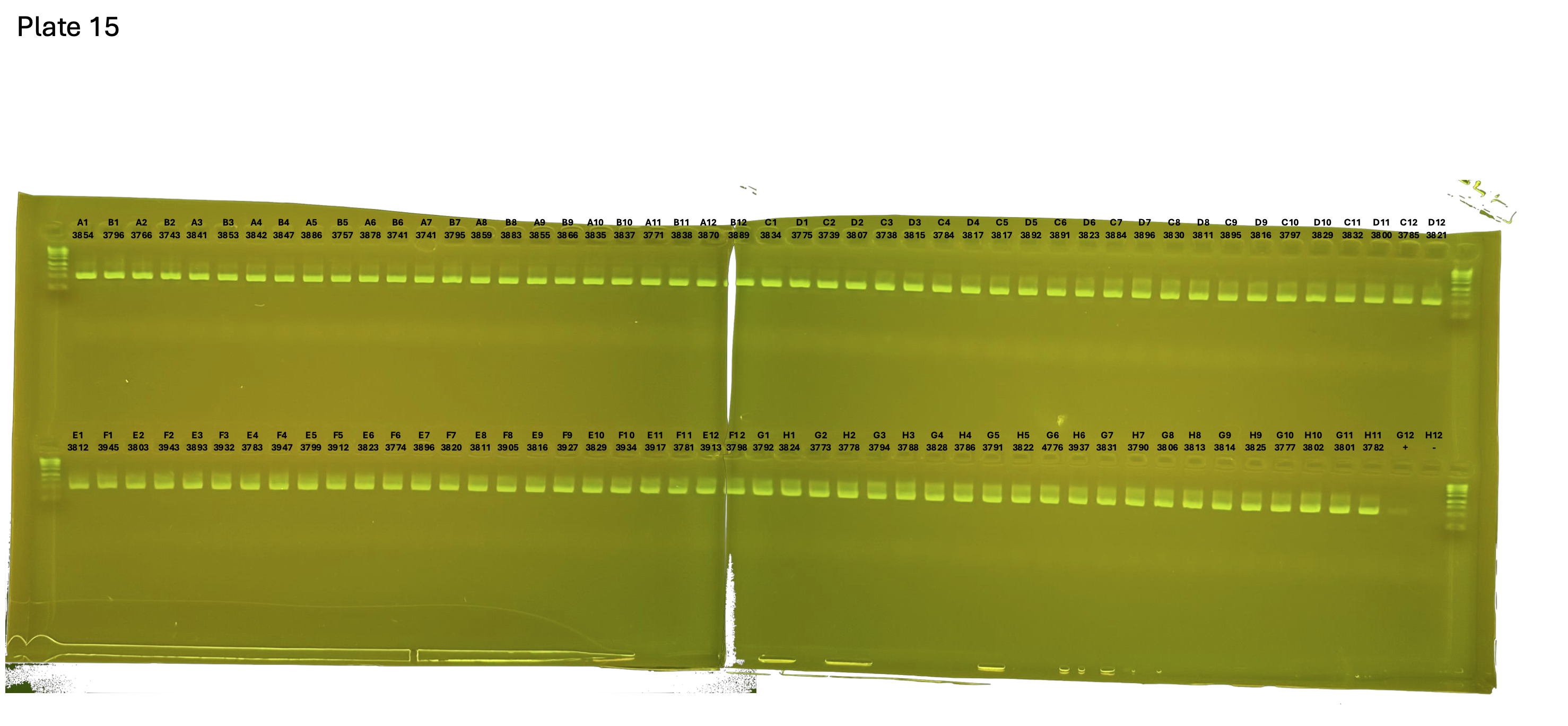
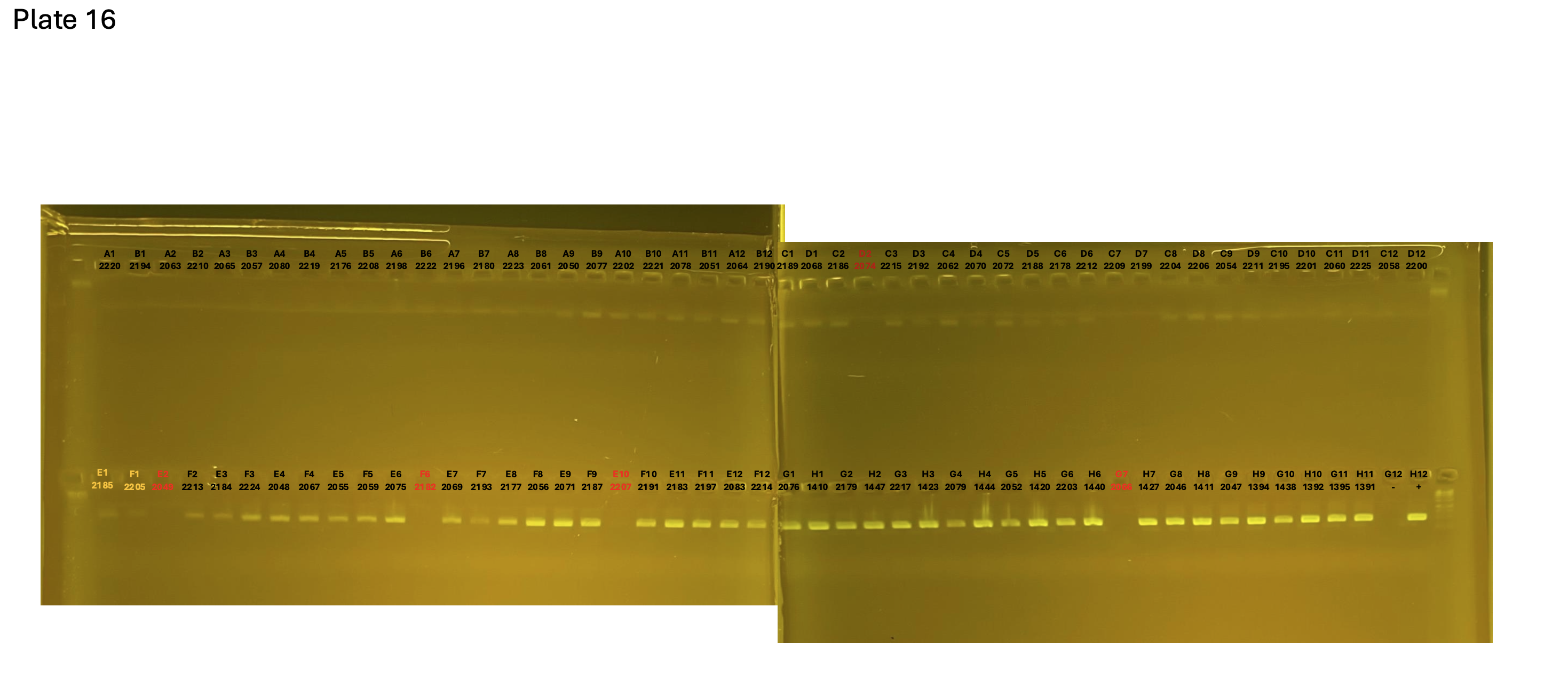
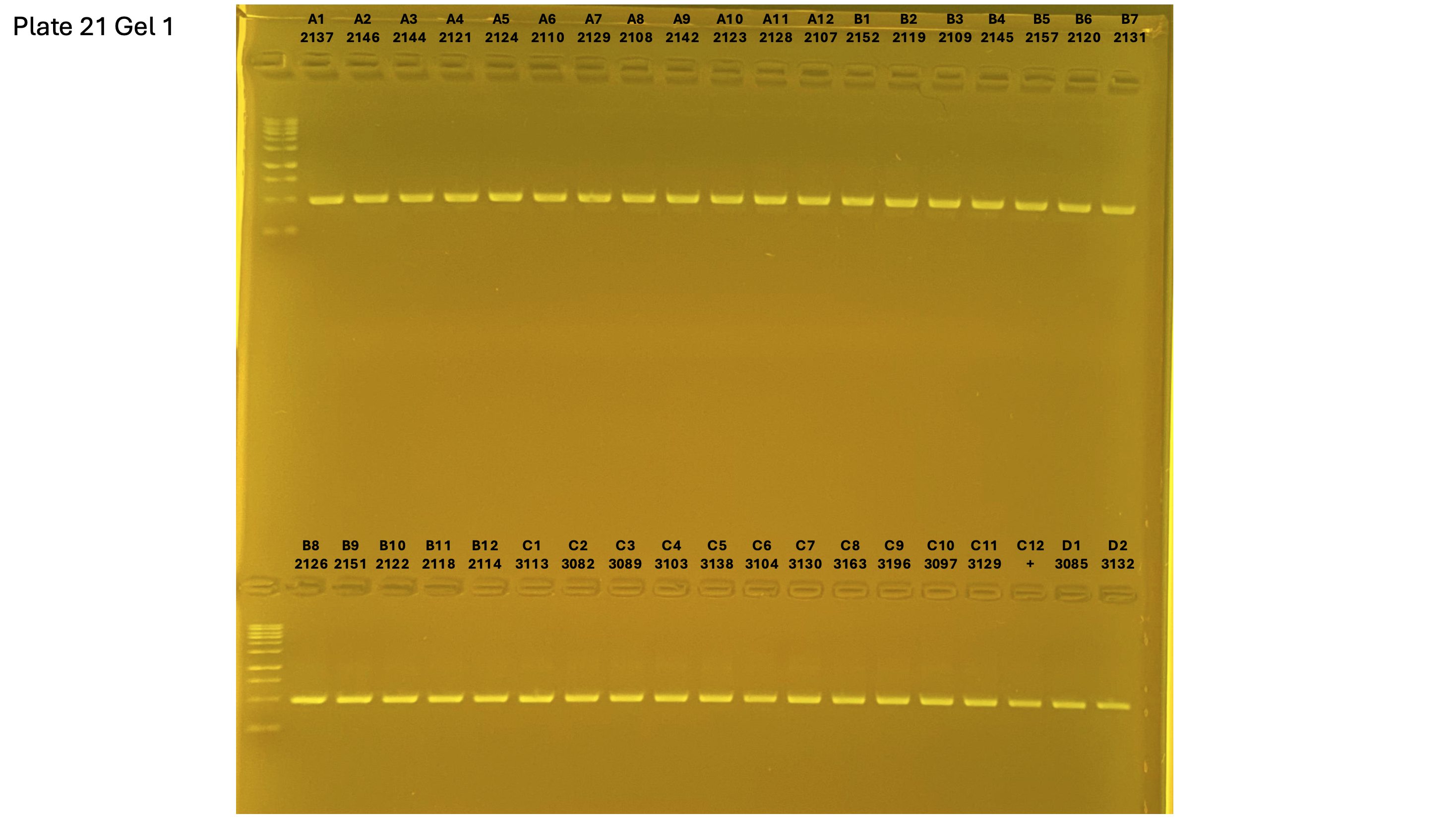
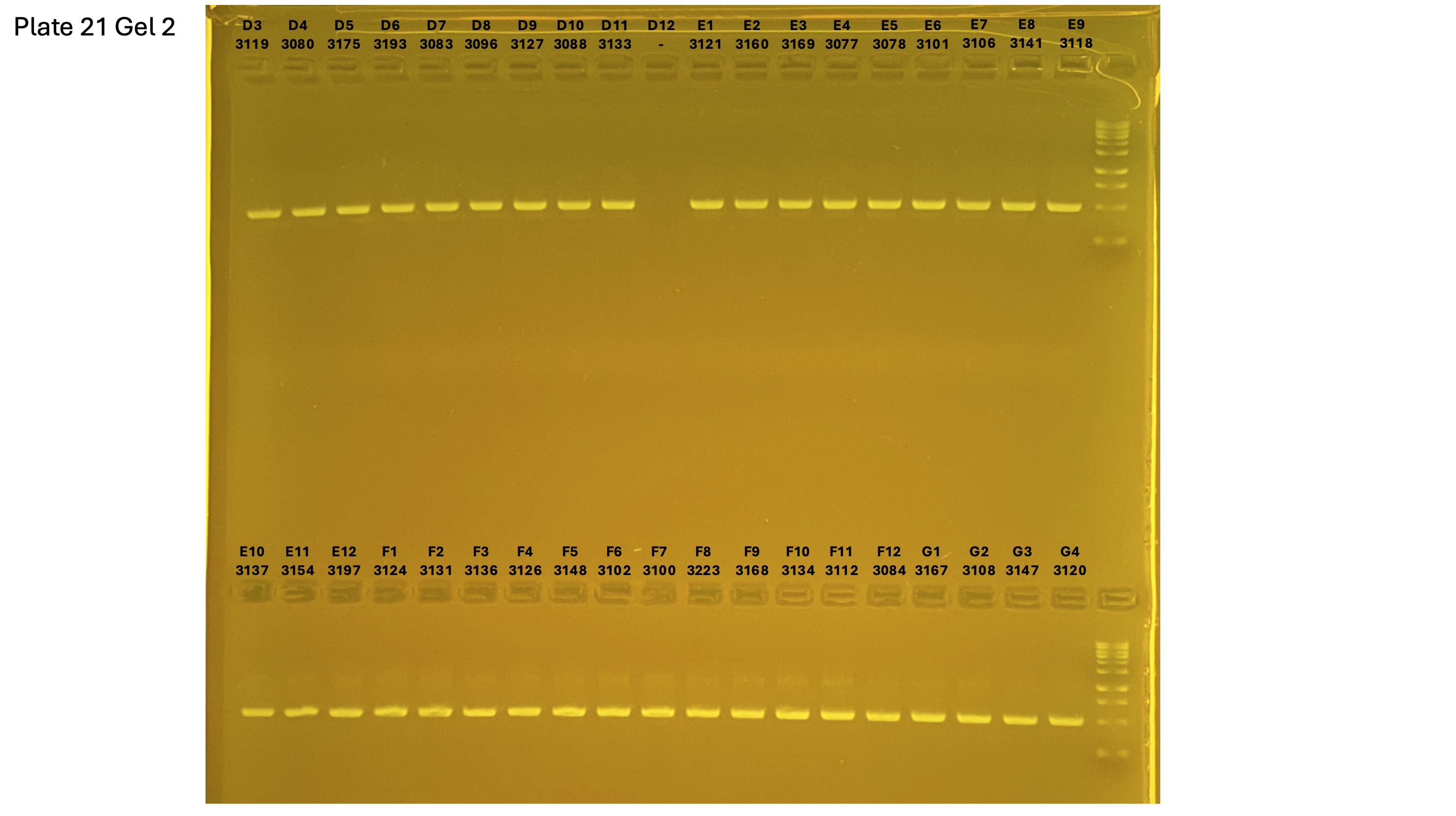
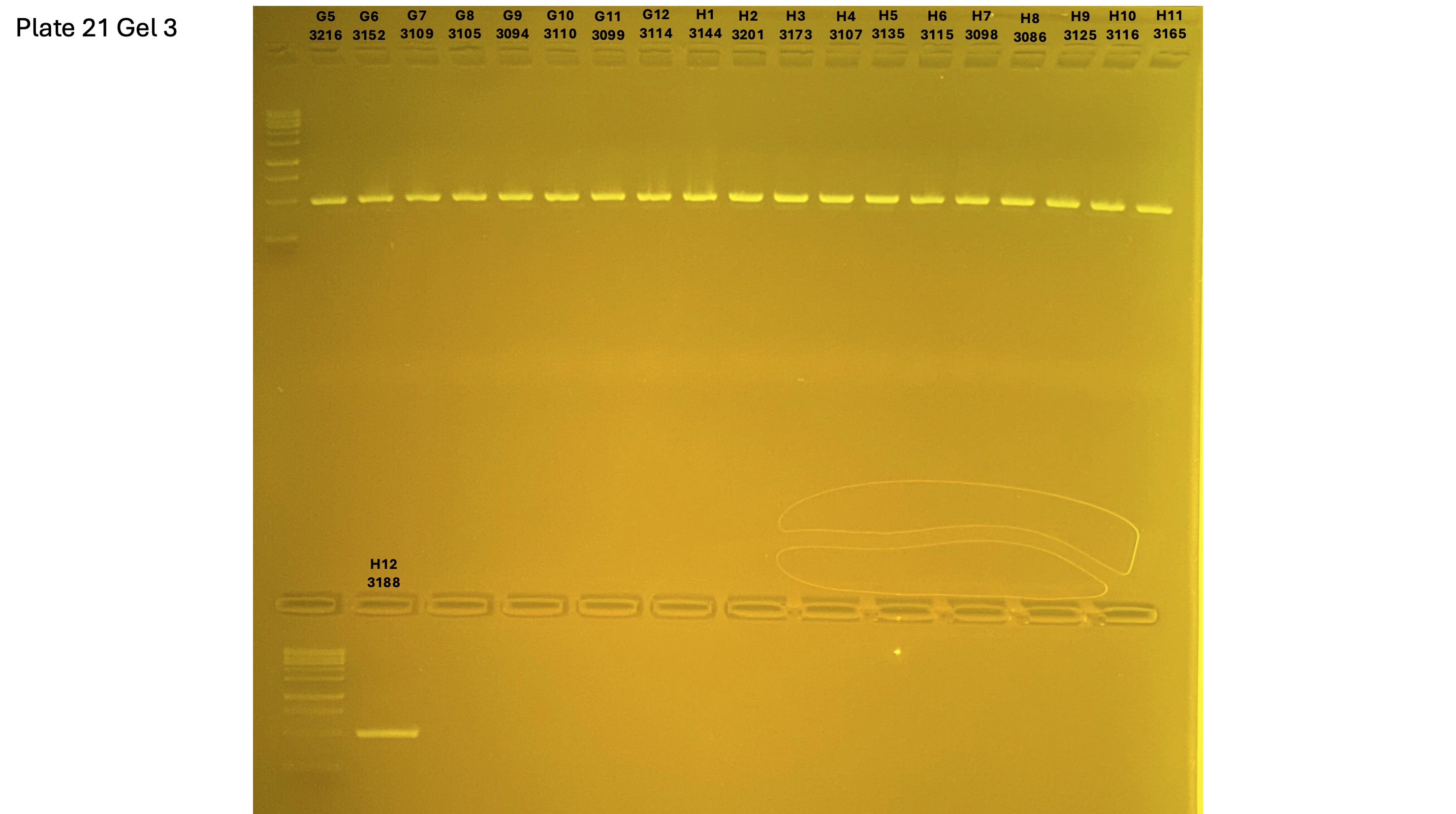
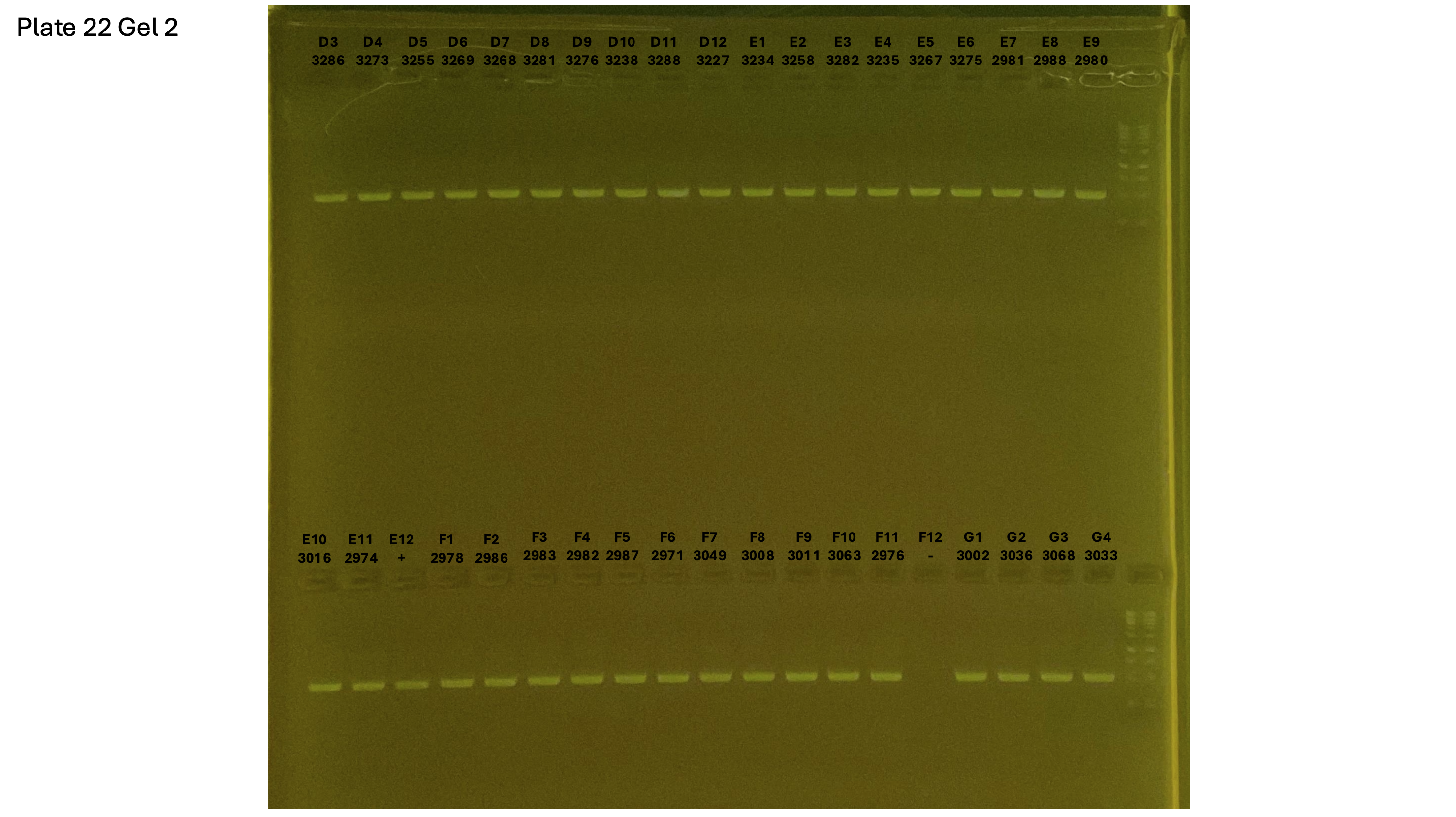
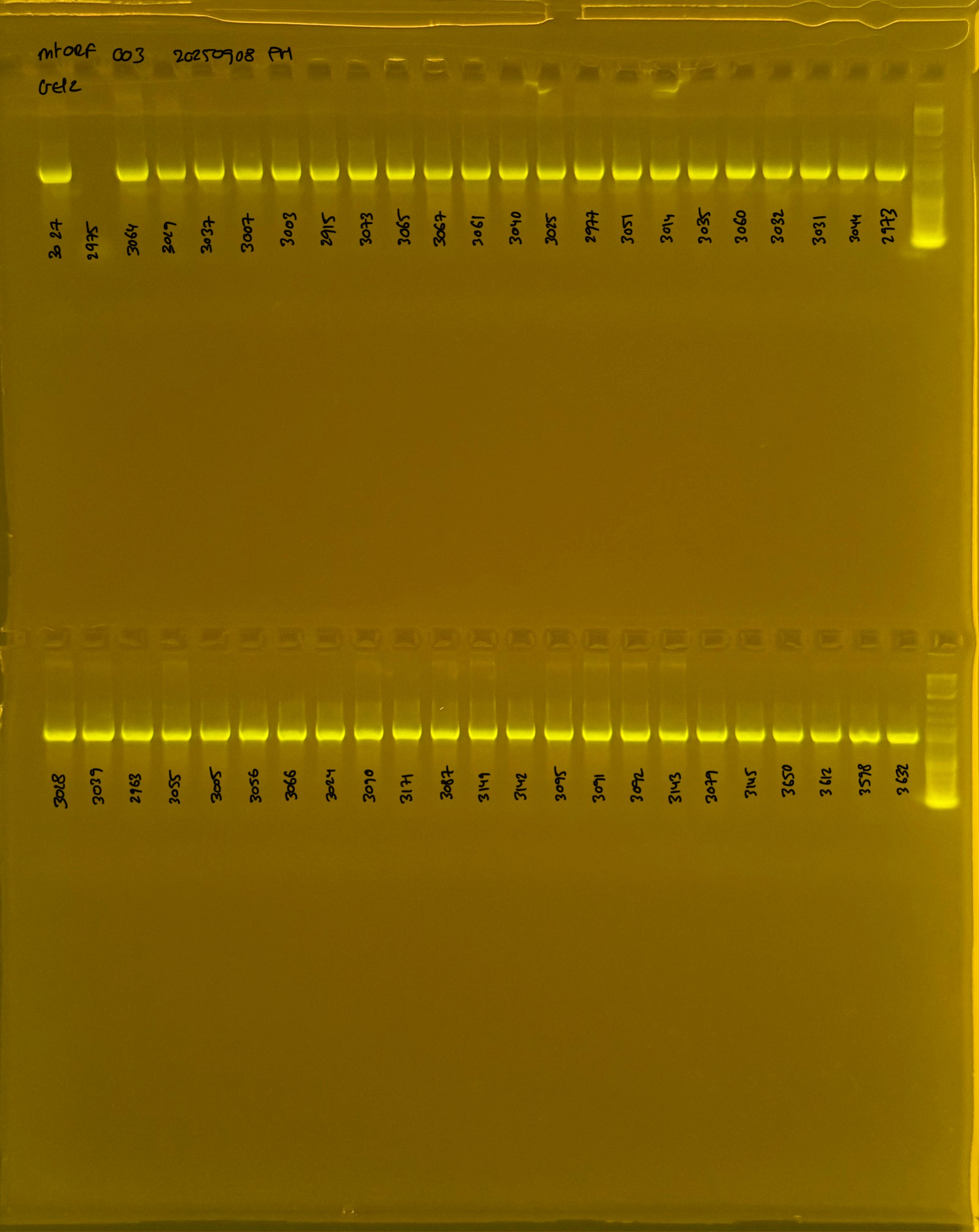
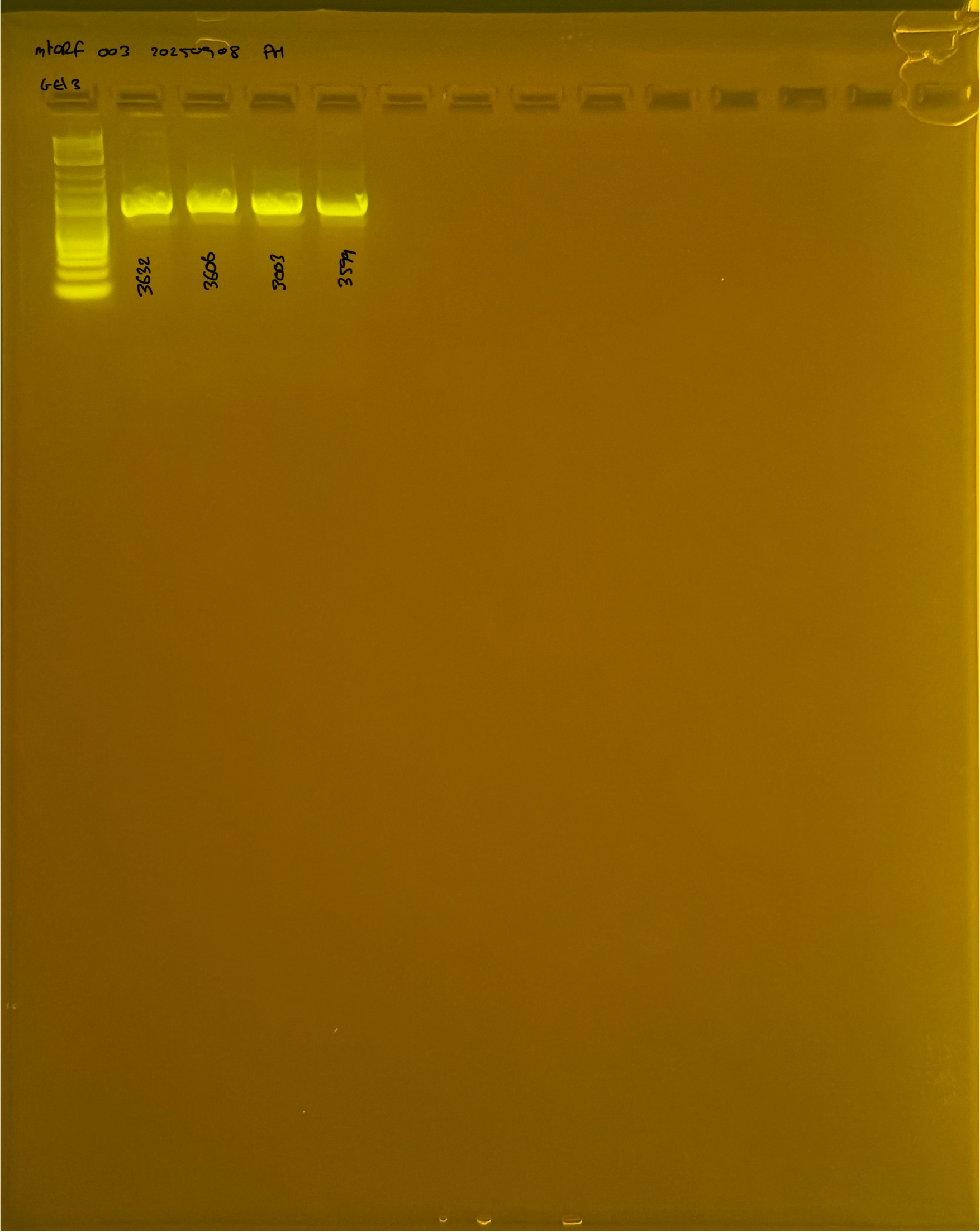
On each gel add 4µl of lader on one side of the gel. On each gel add 4µl of samples in each well. Run the gel at 100V during 35 minutes.
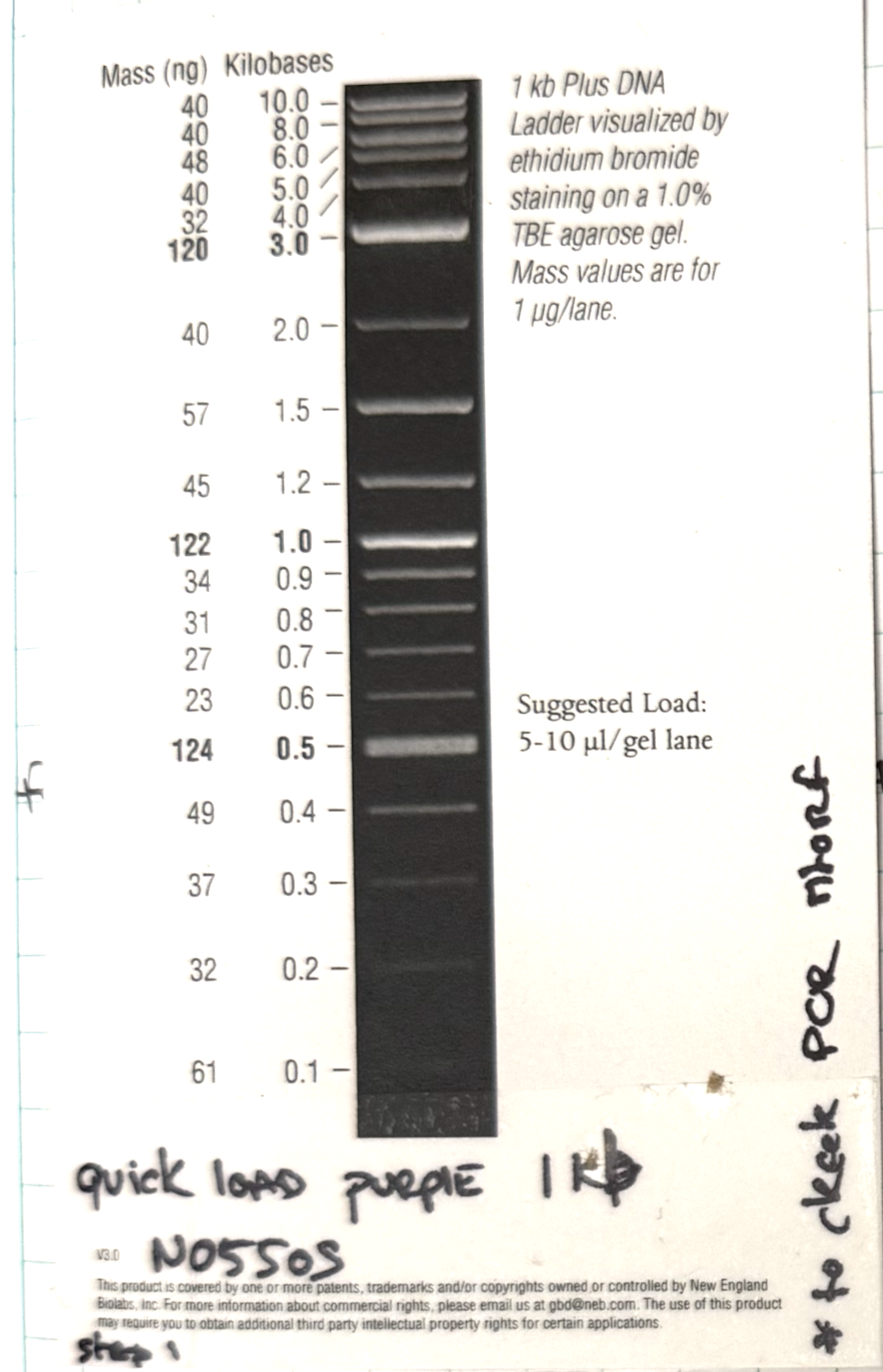
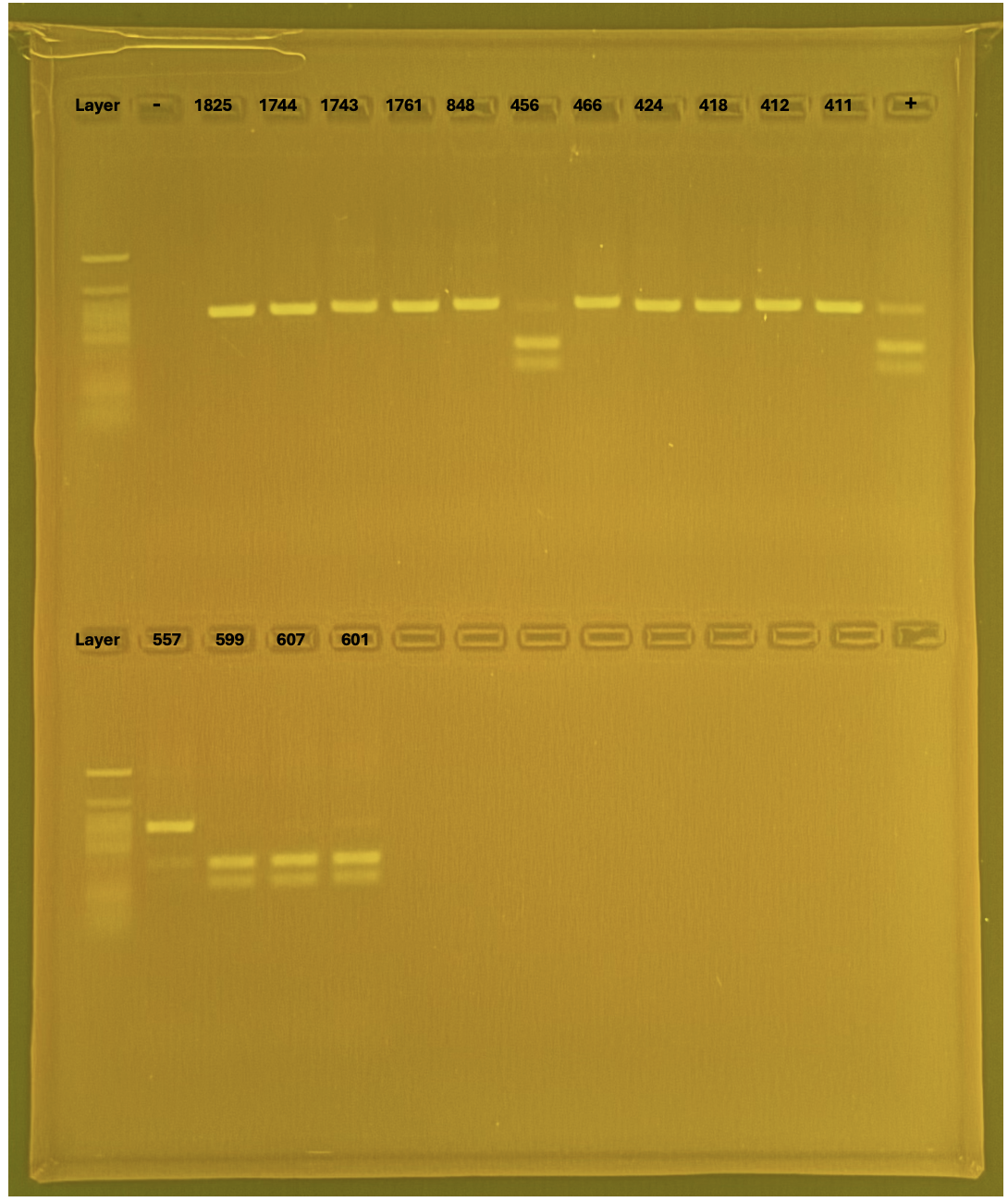
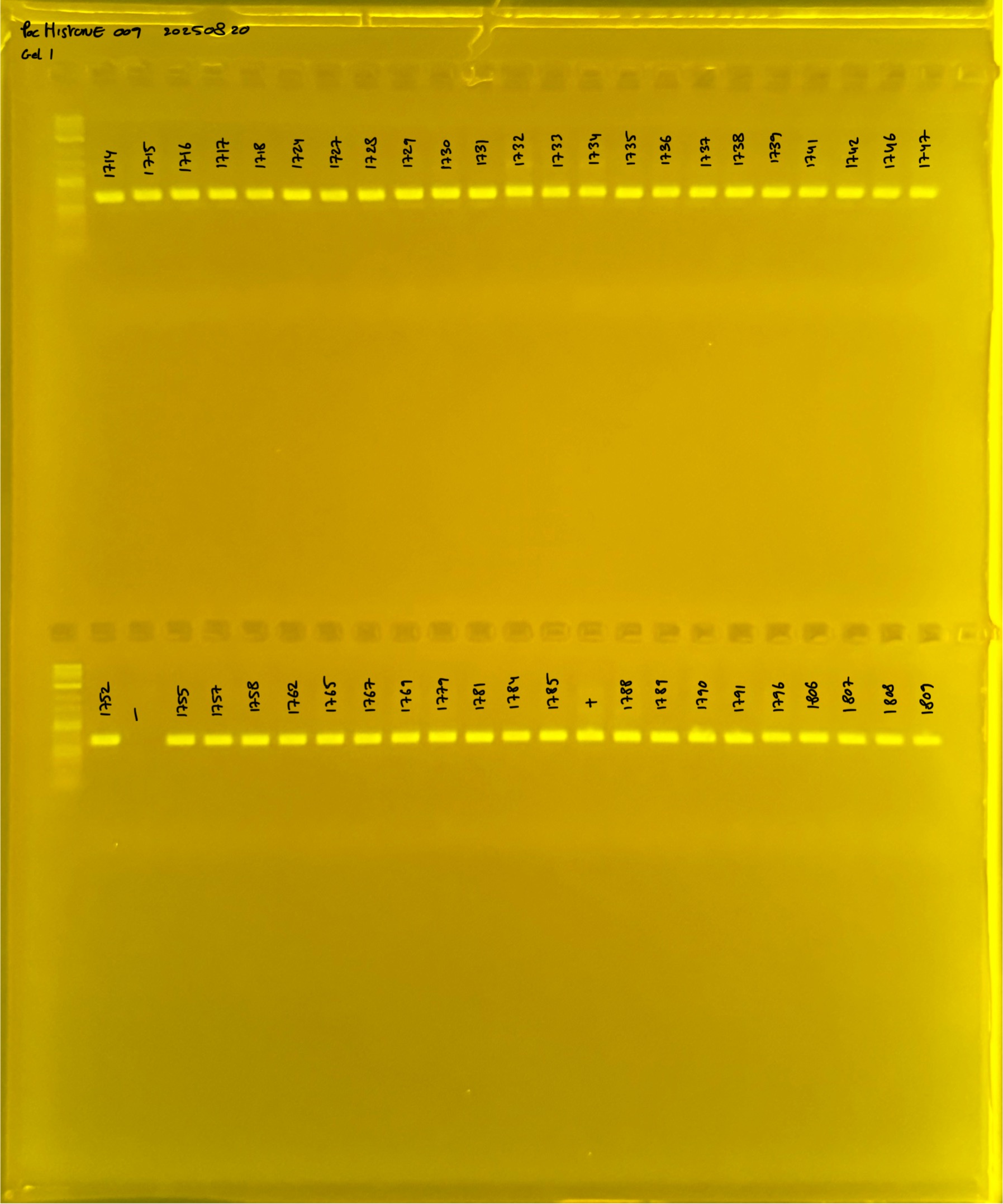
Layer use for mtORF
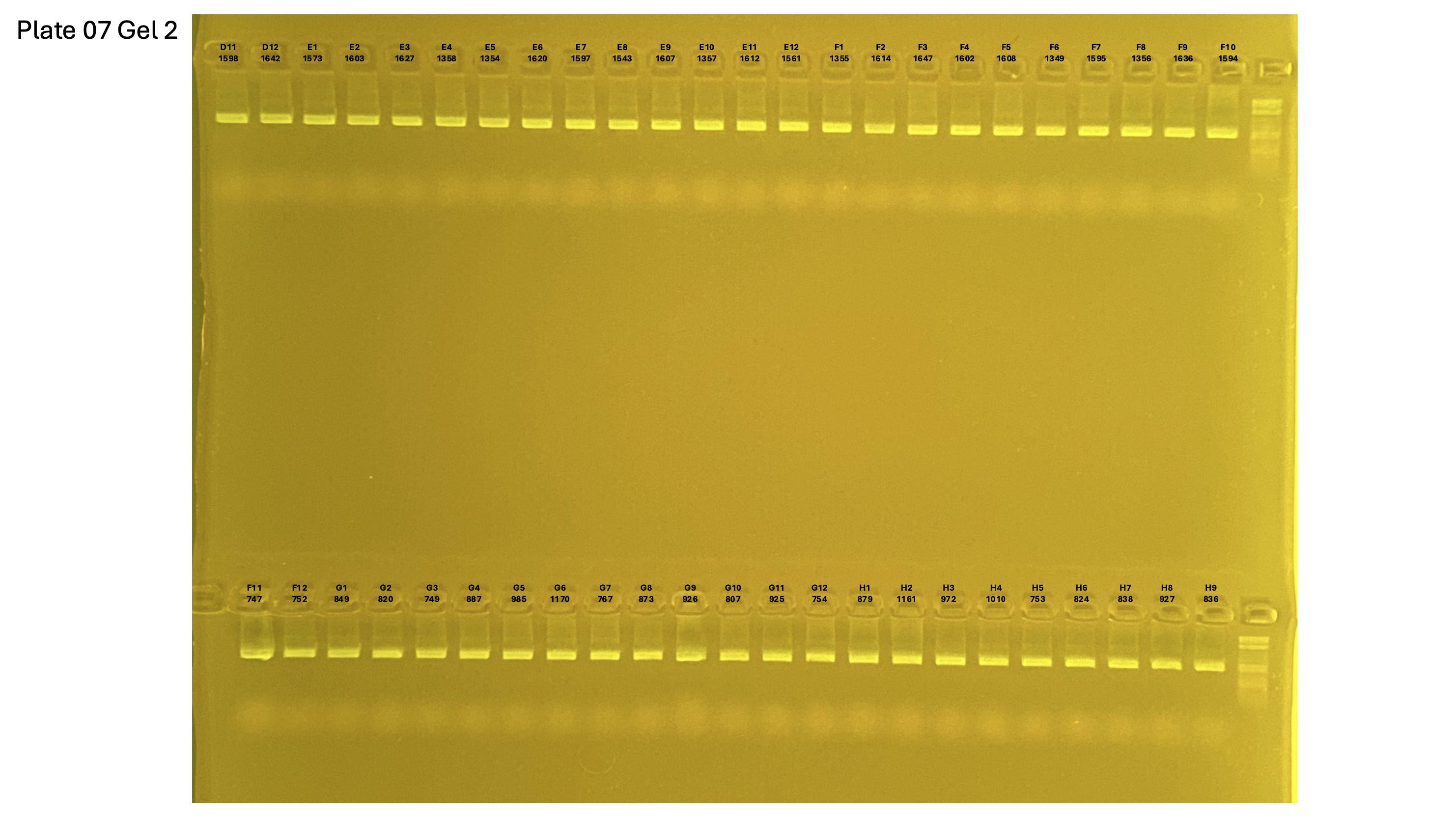
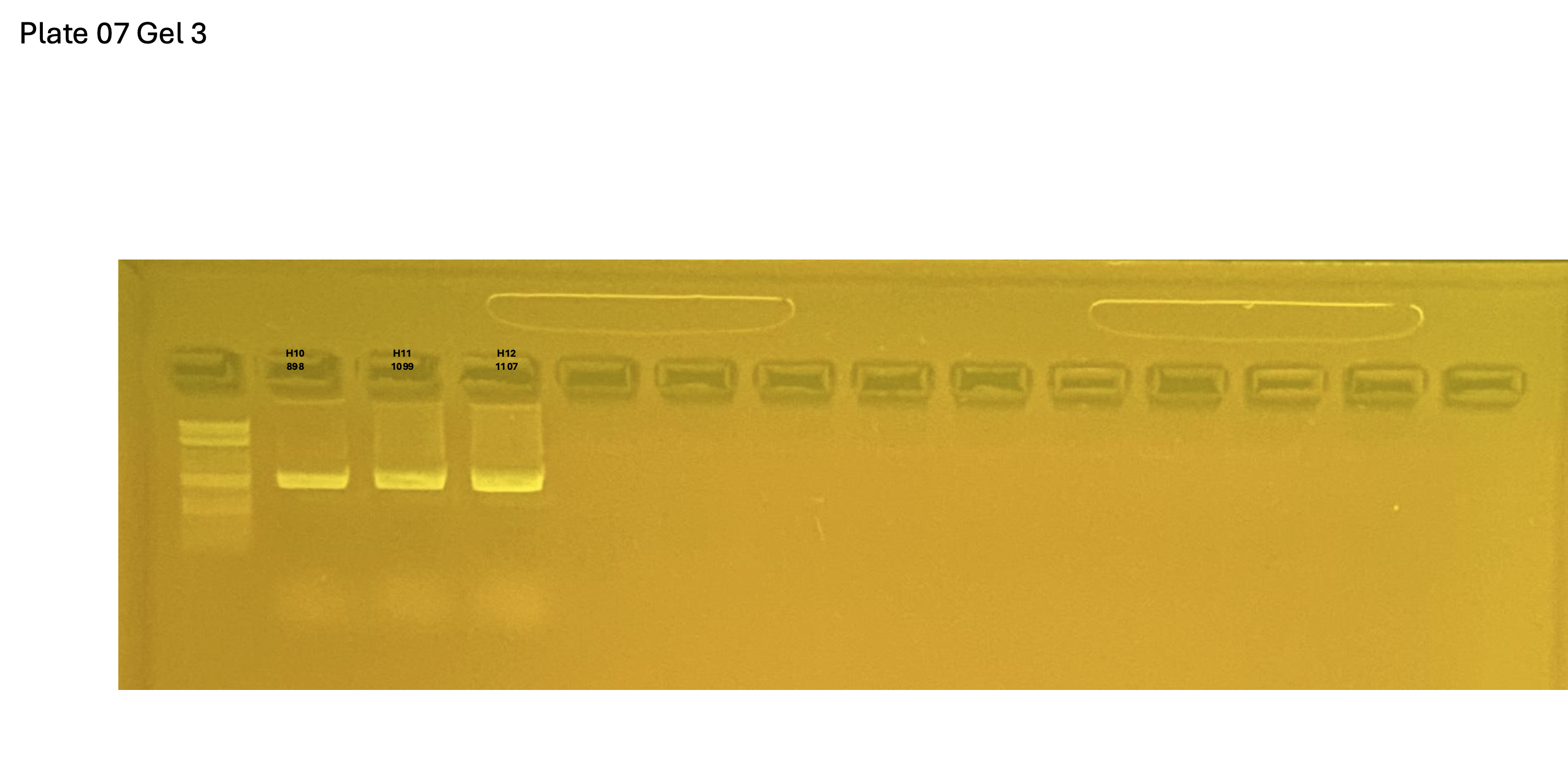
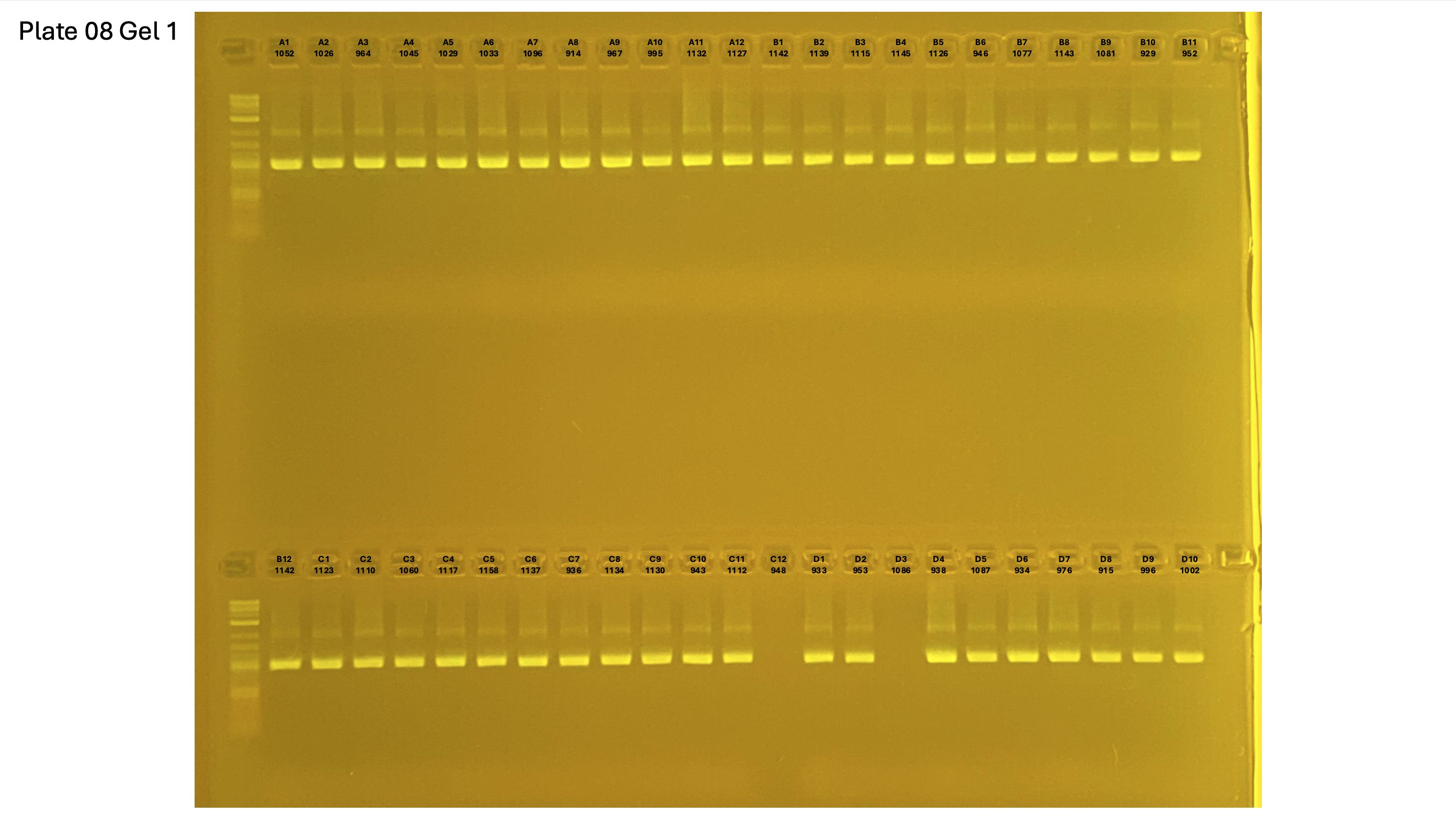
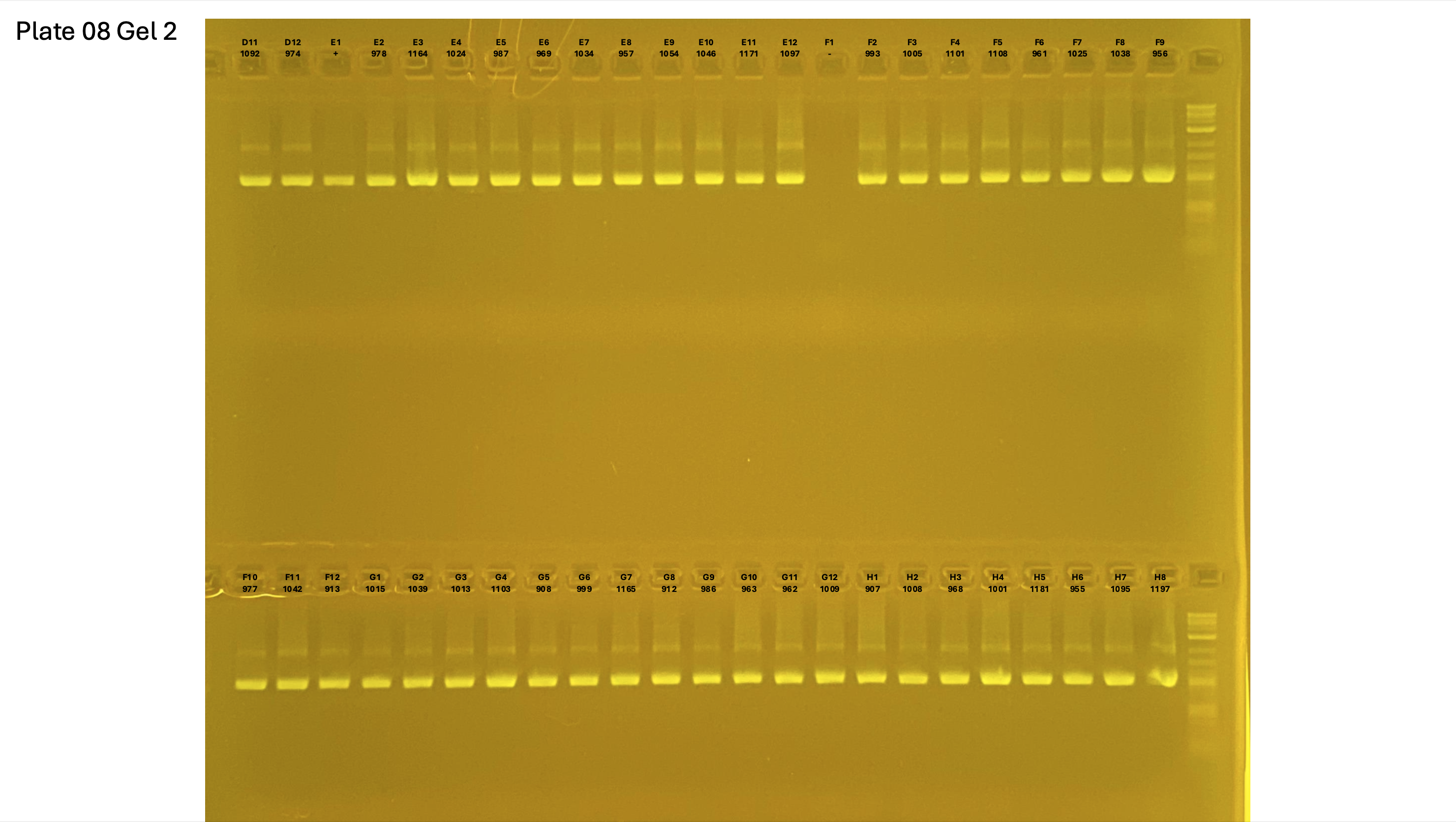
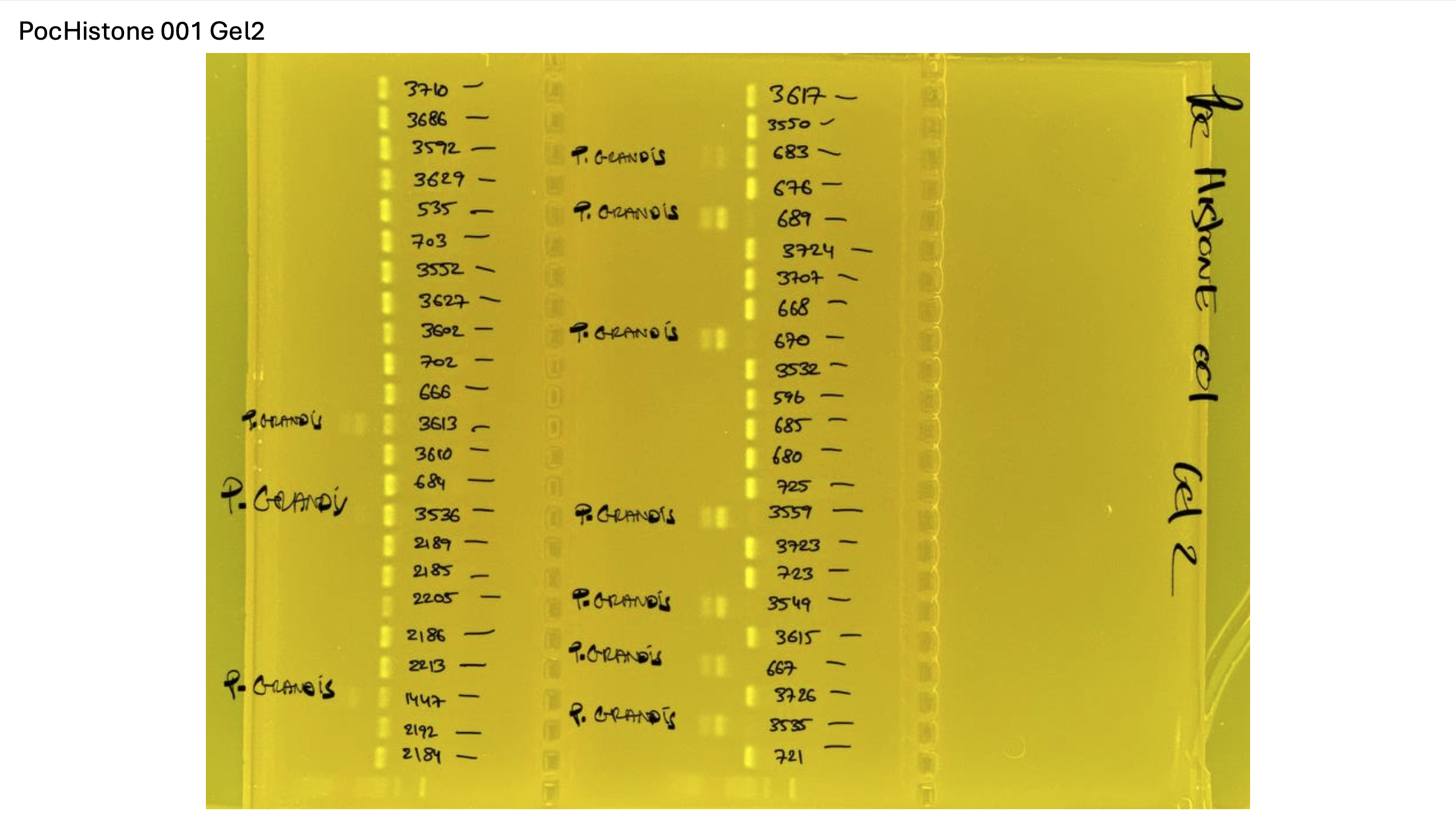
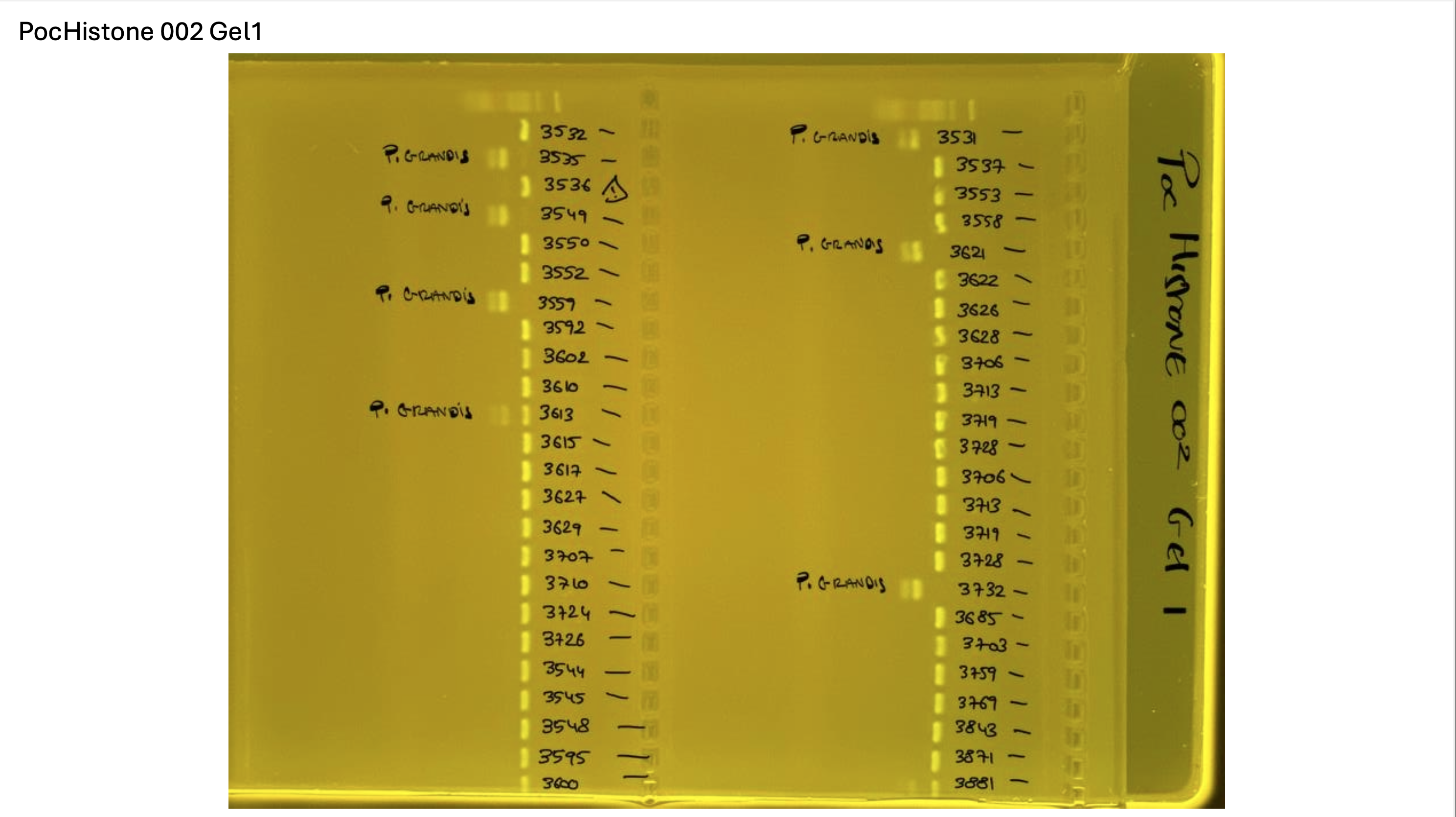
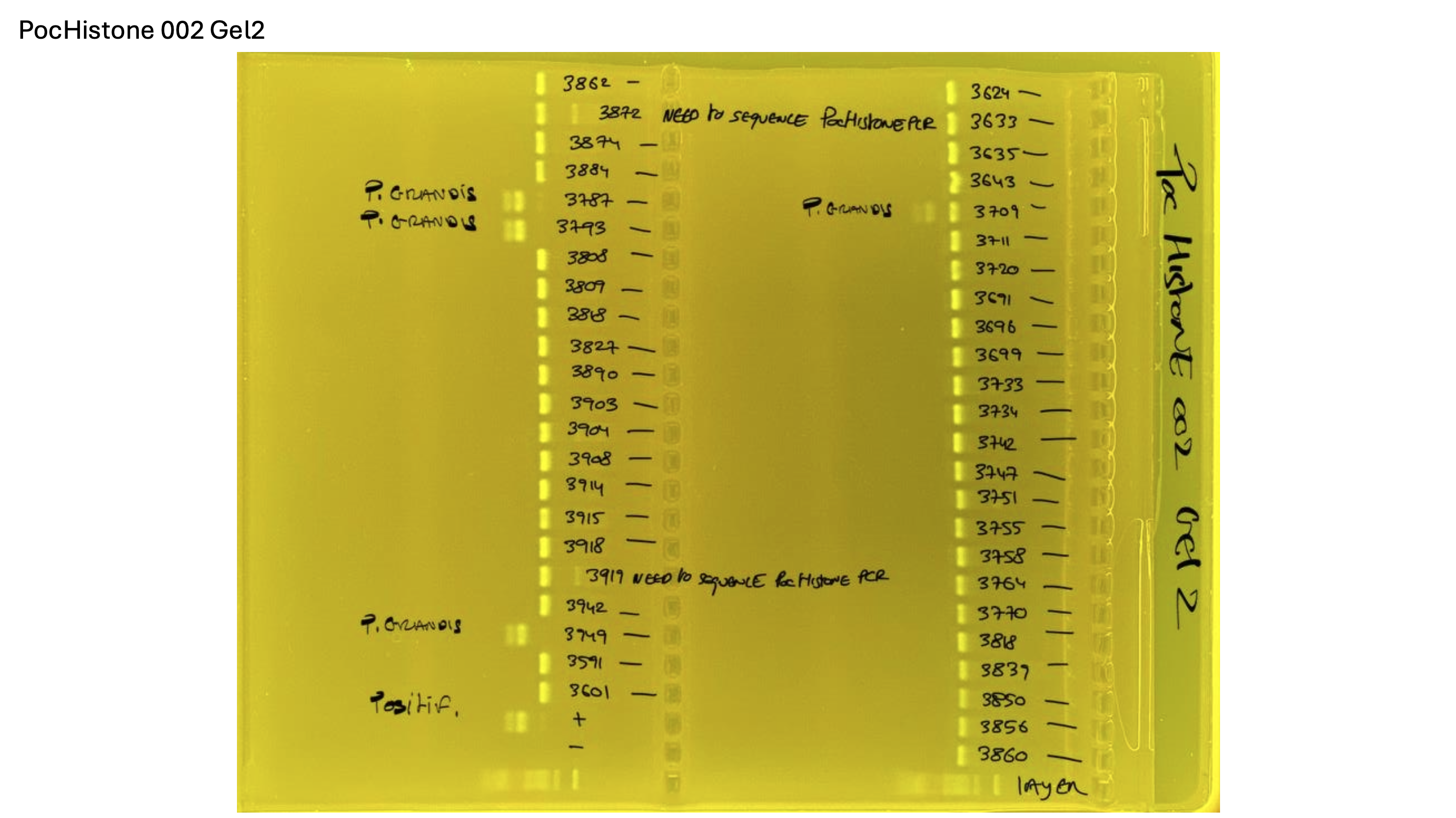
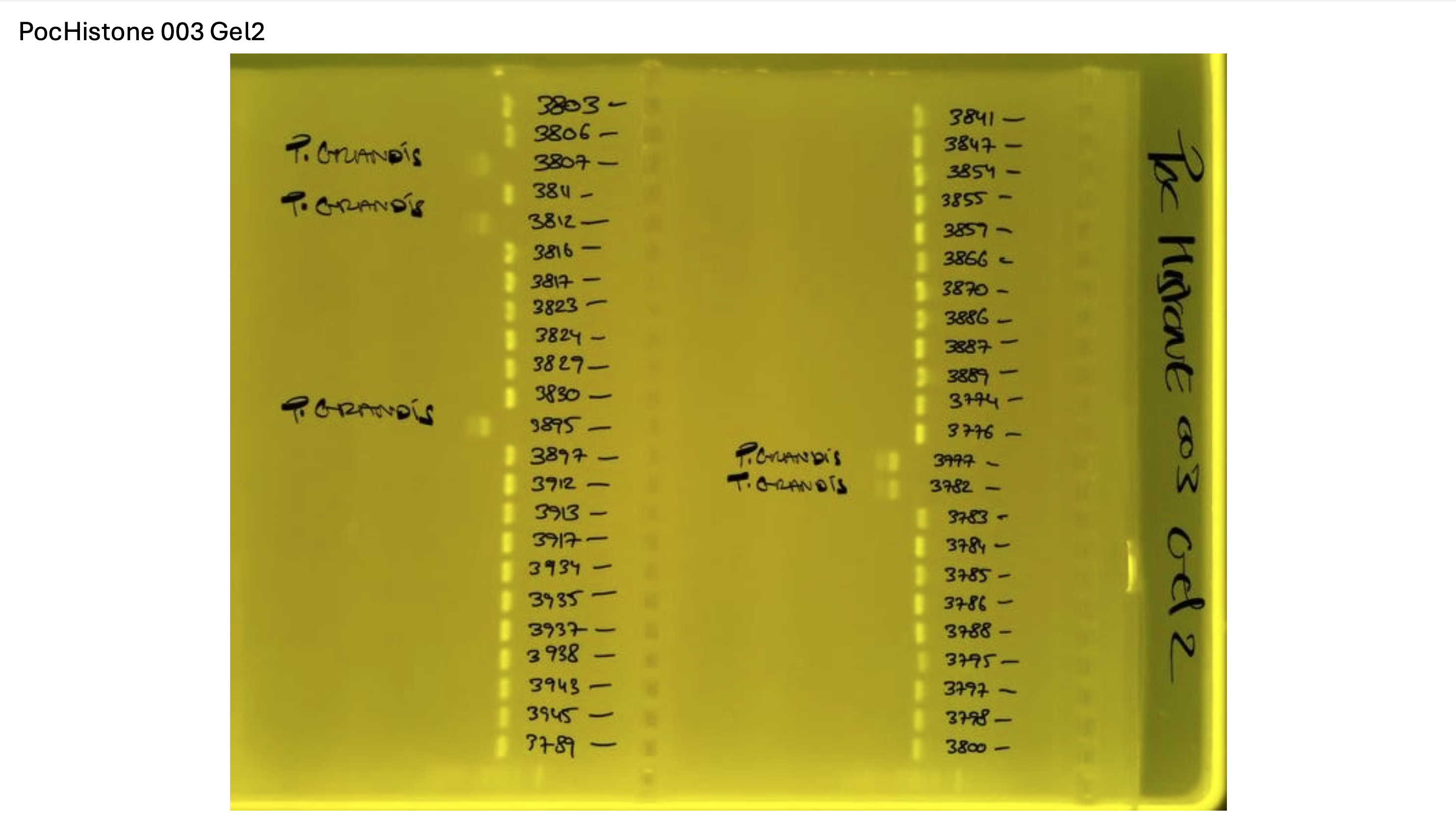
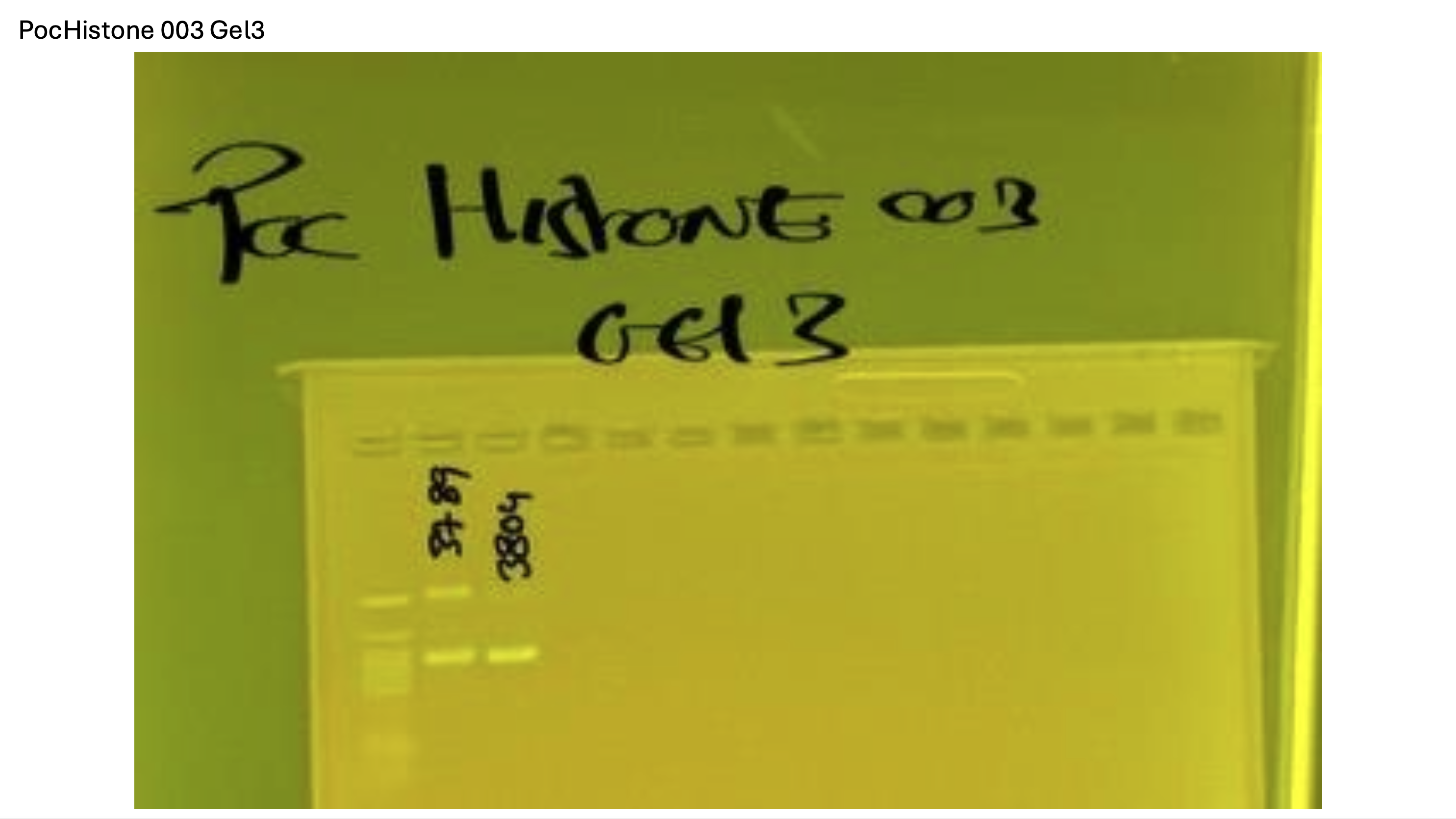
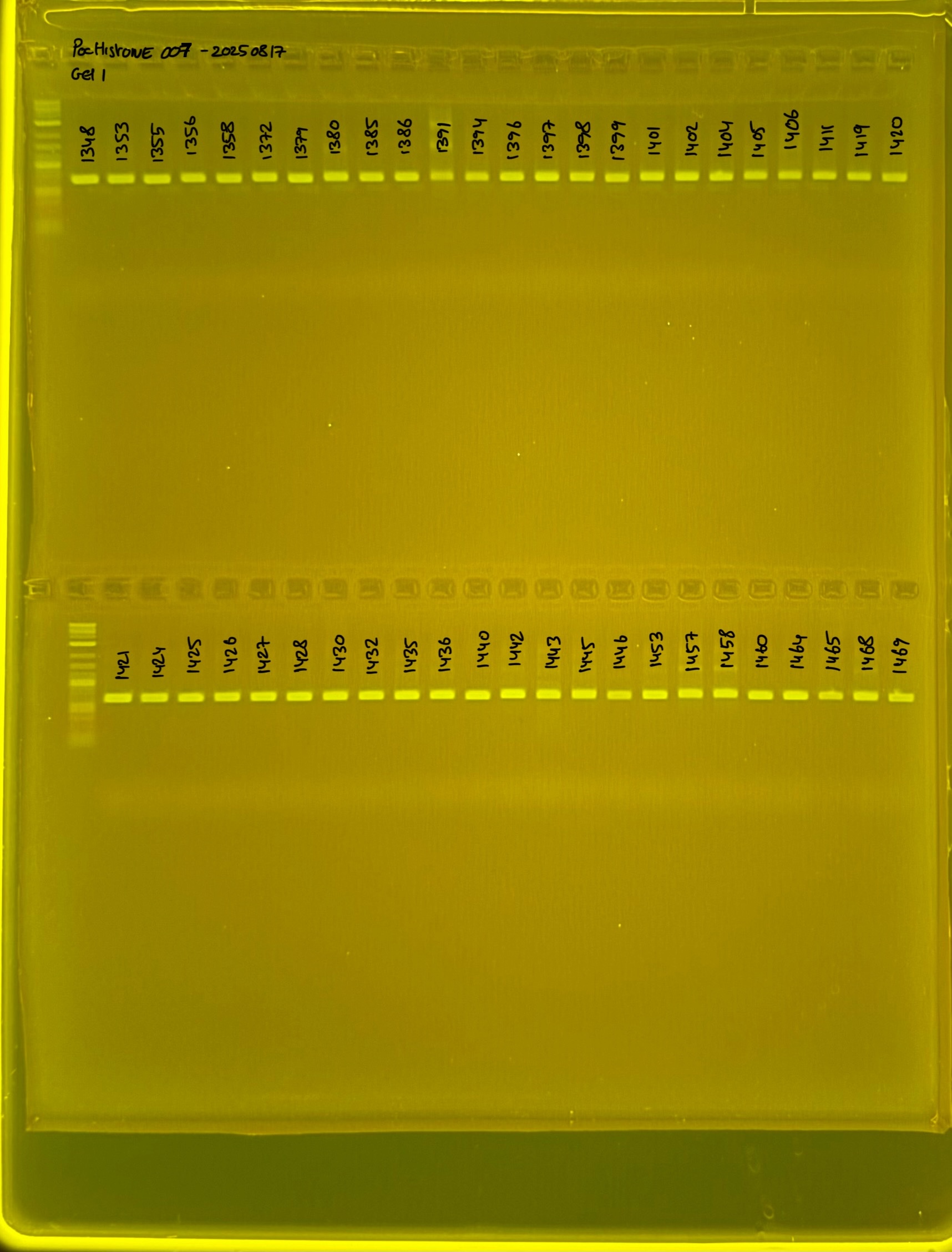
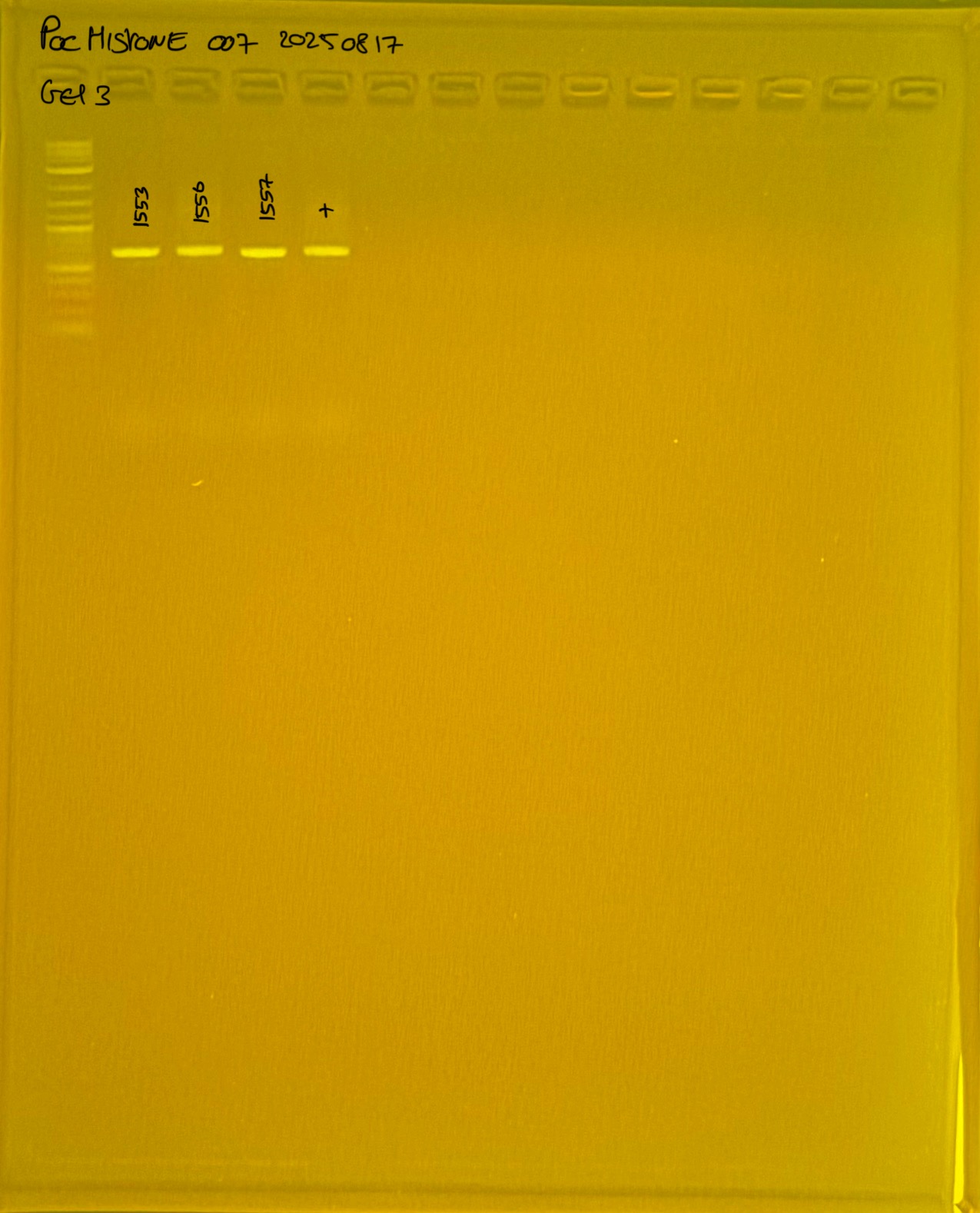
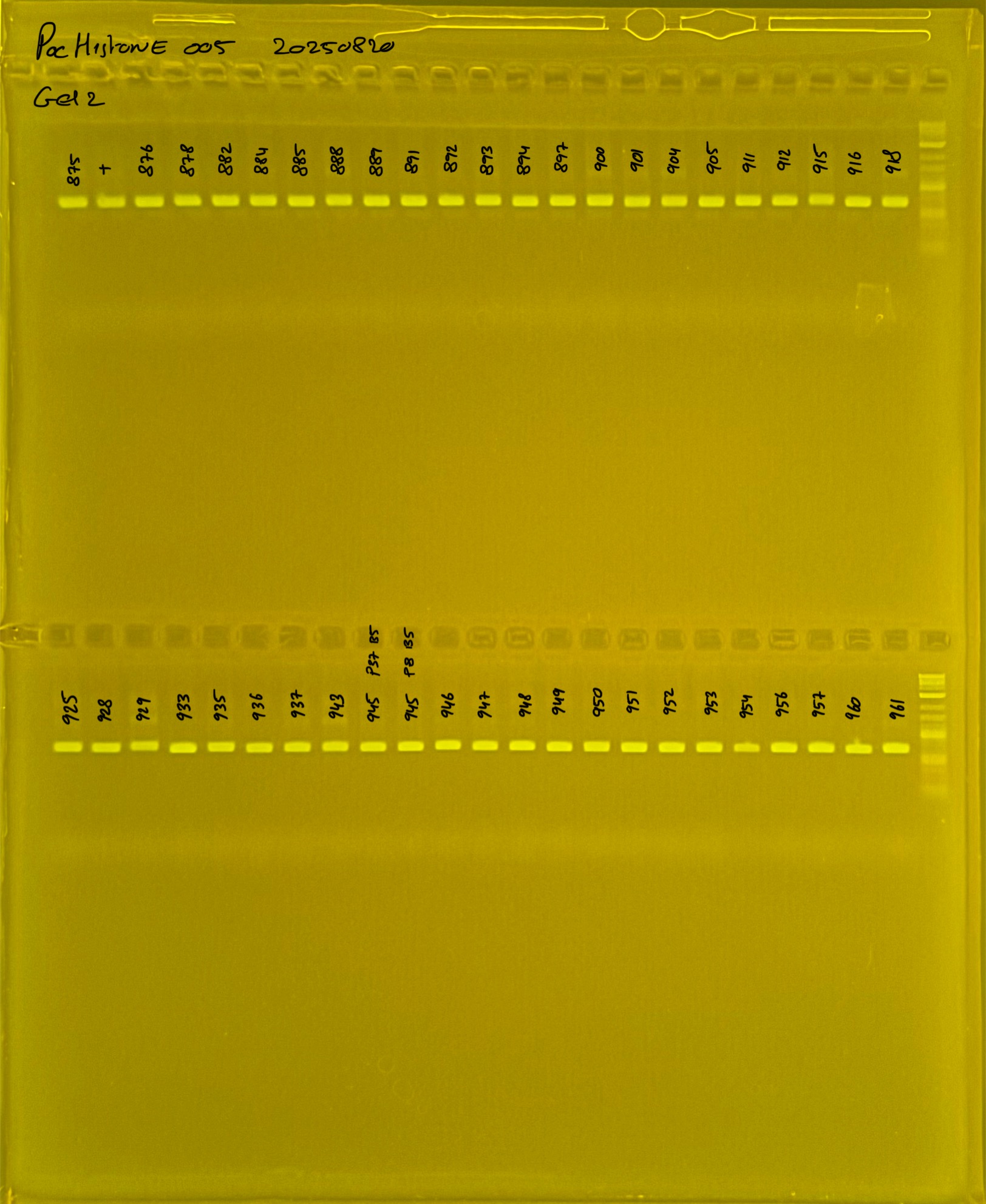
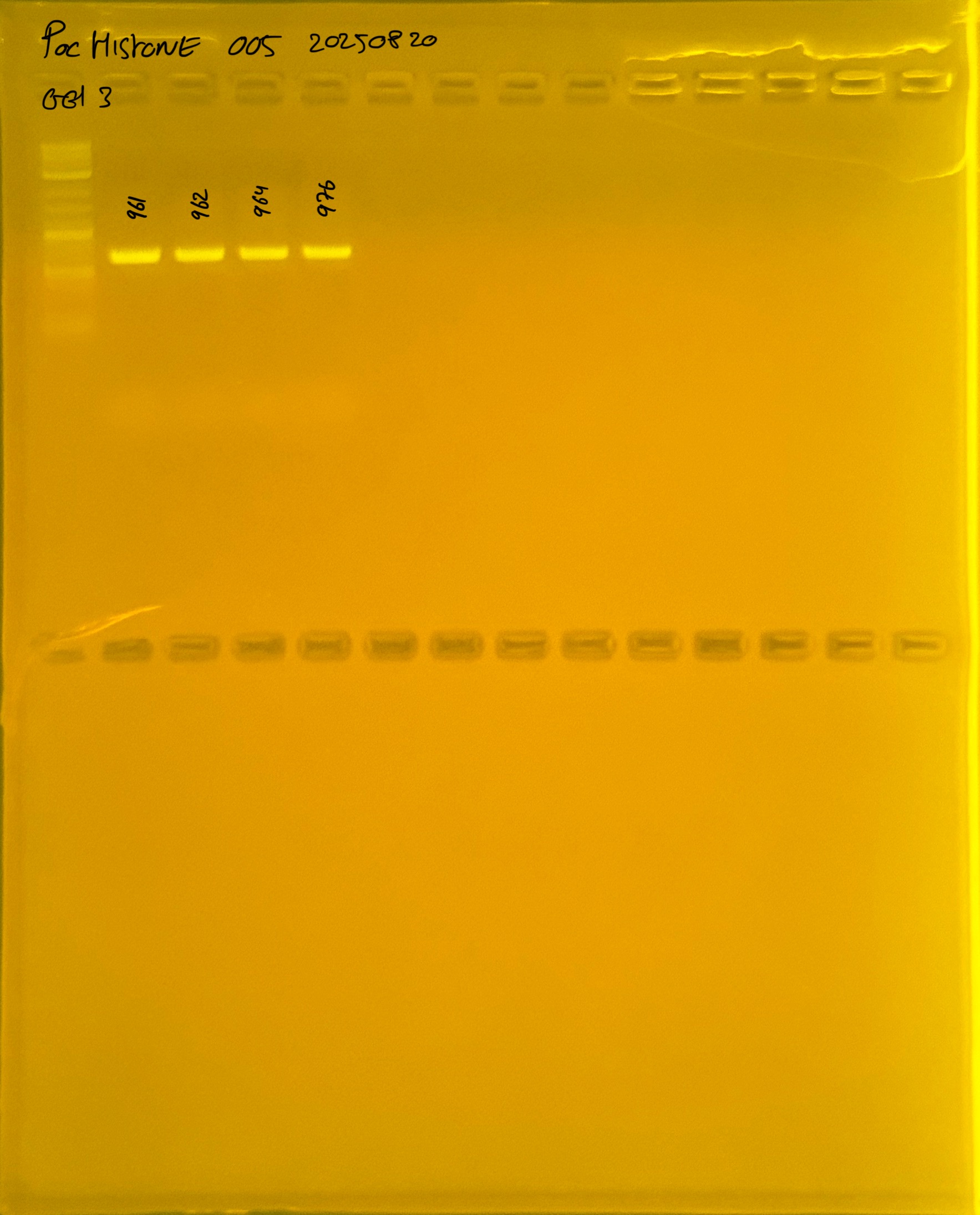
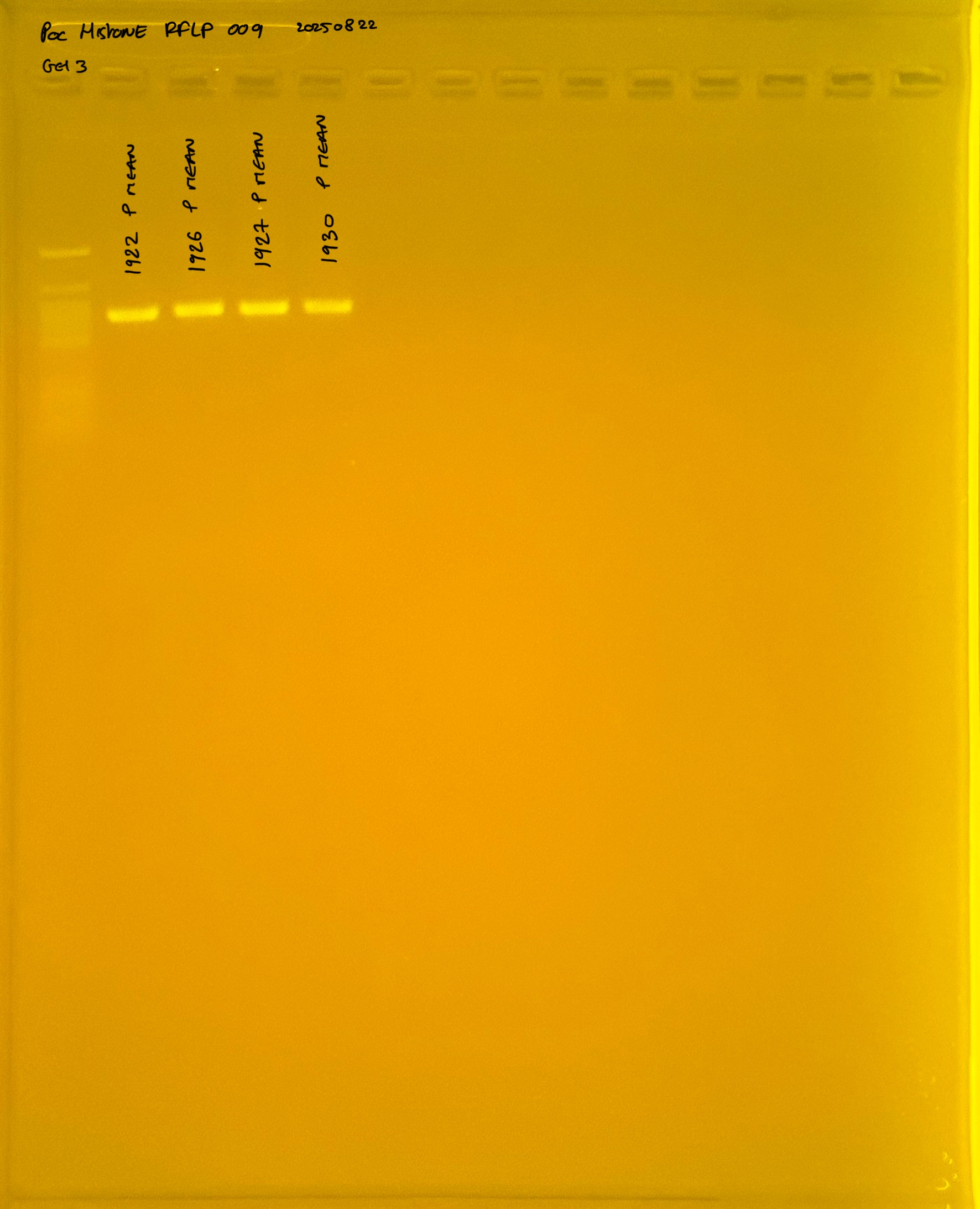
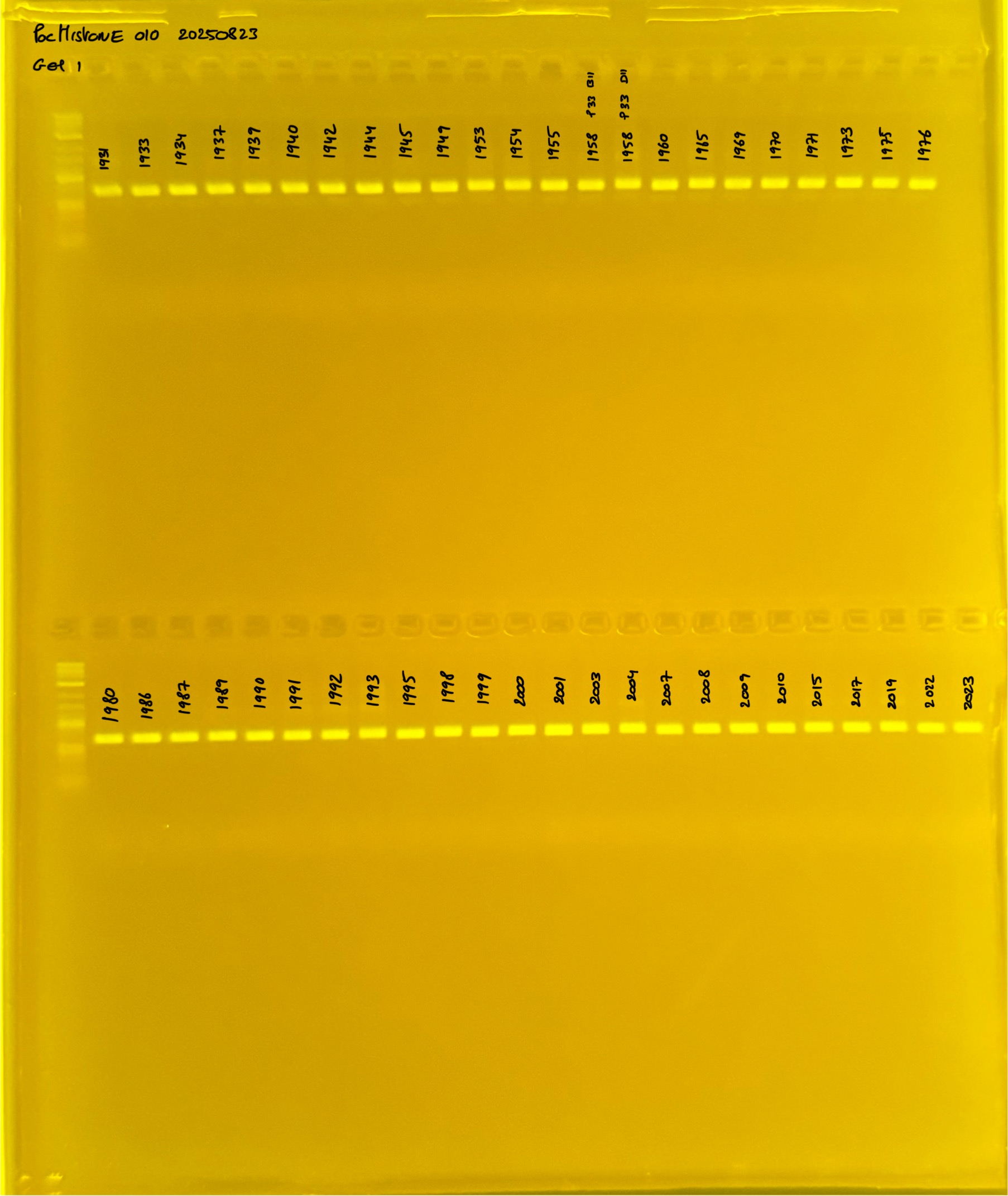
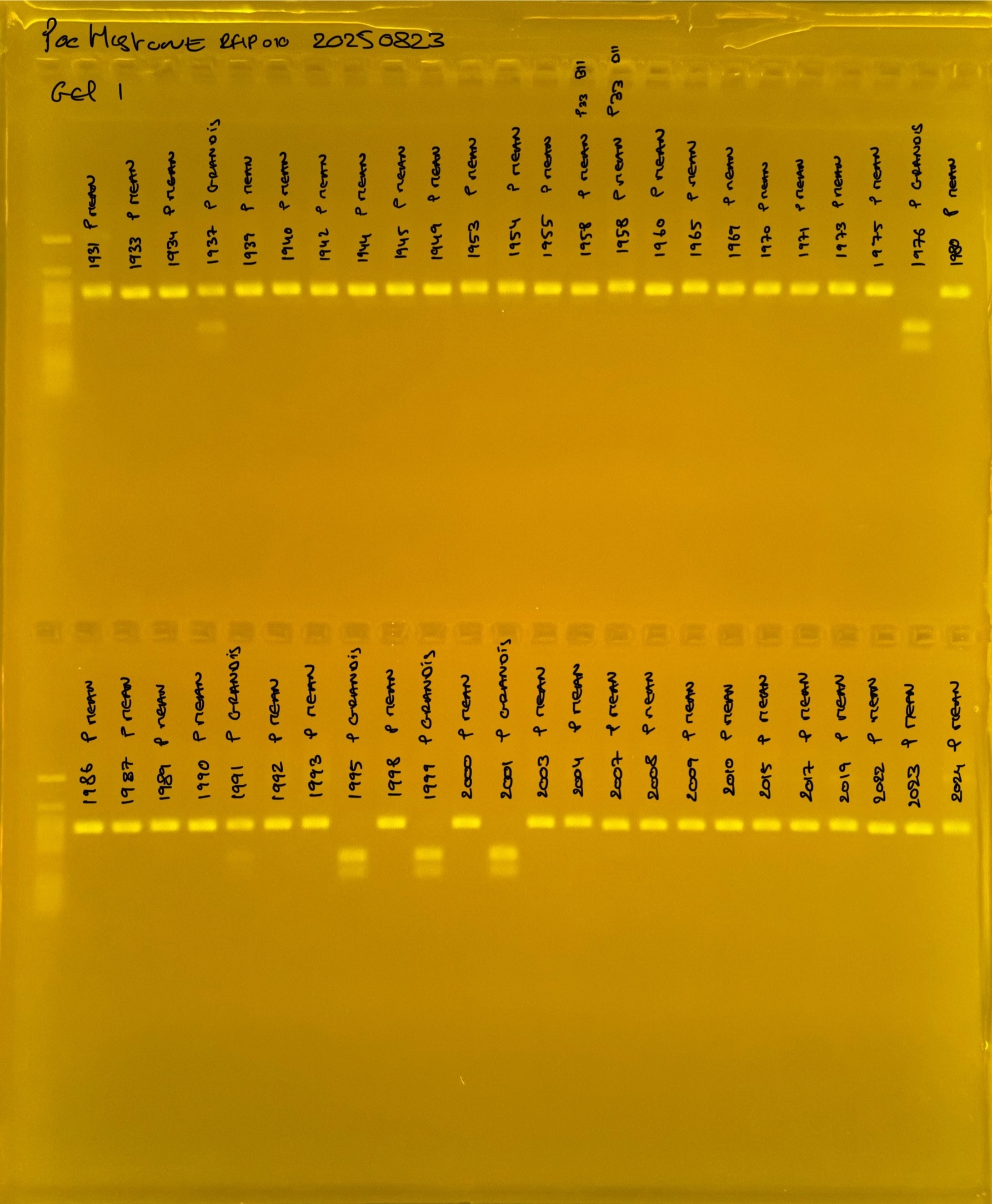
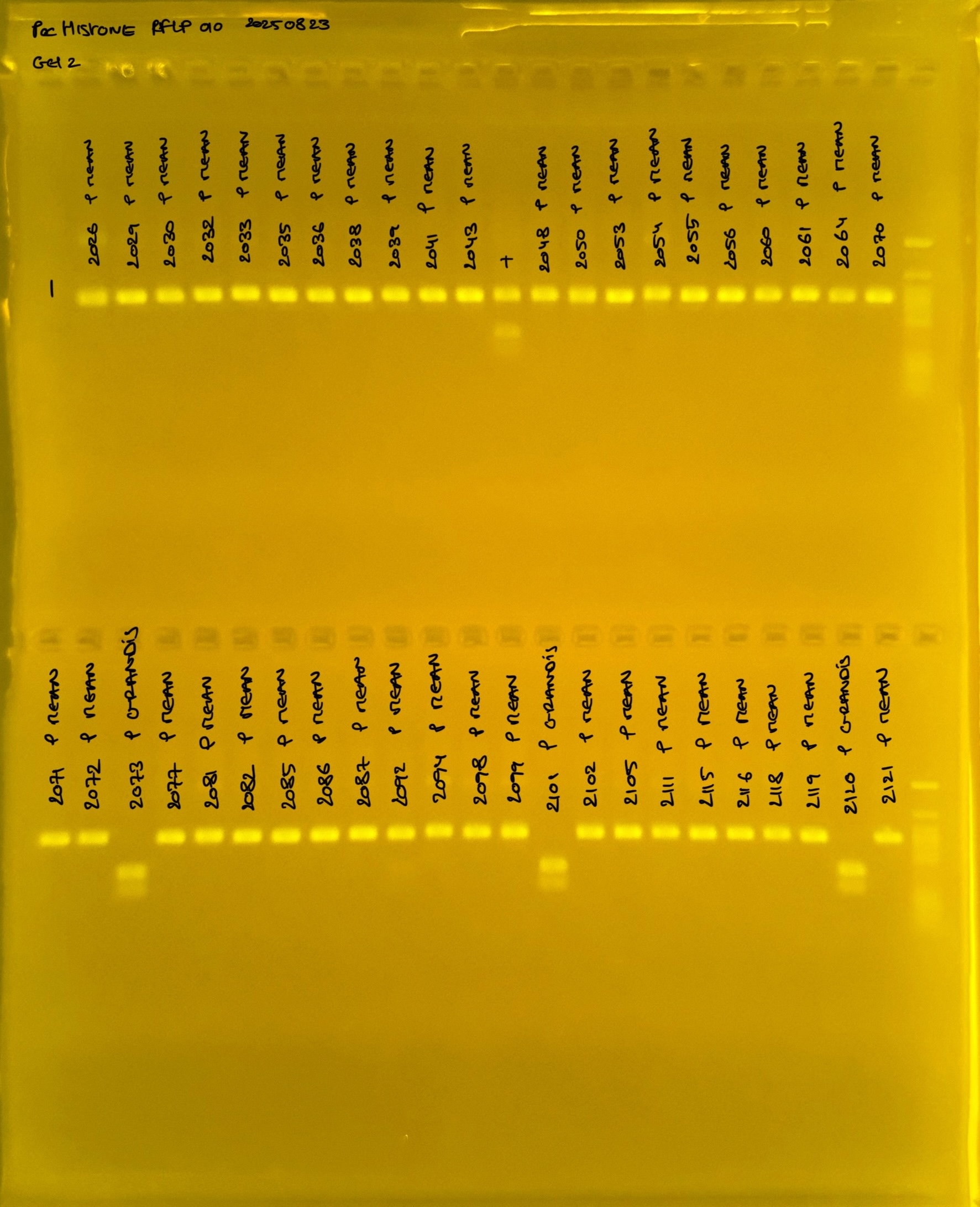
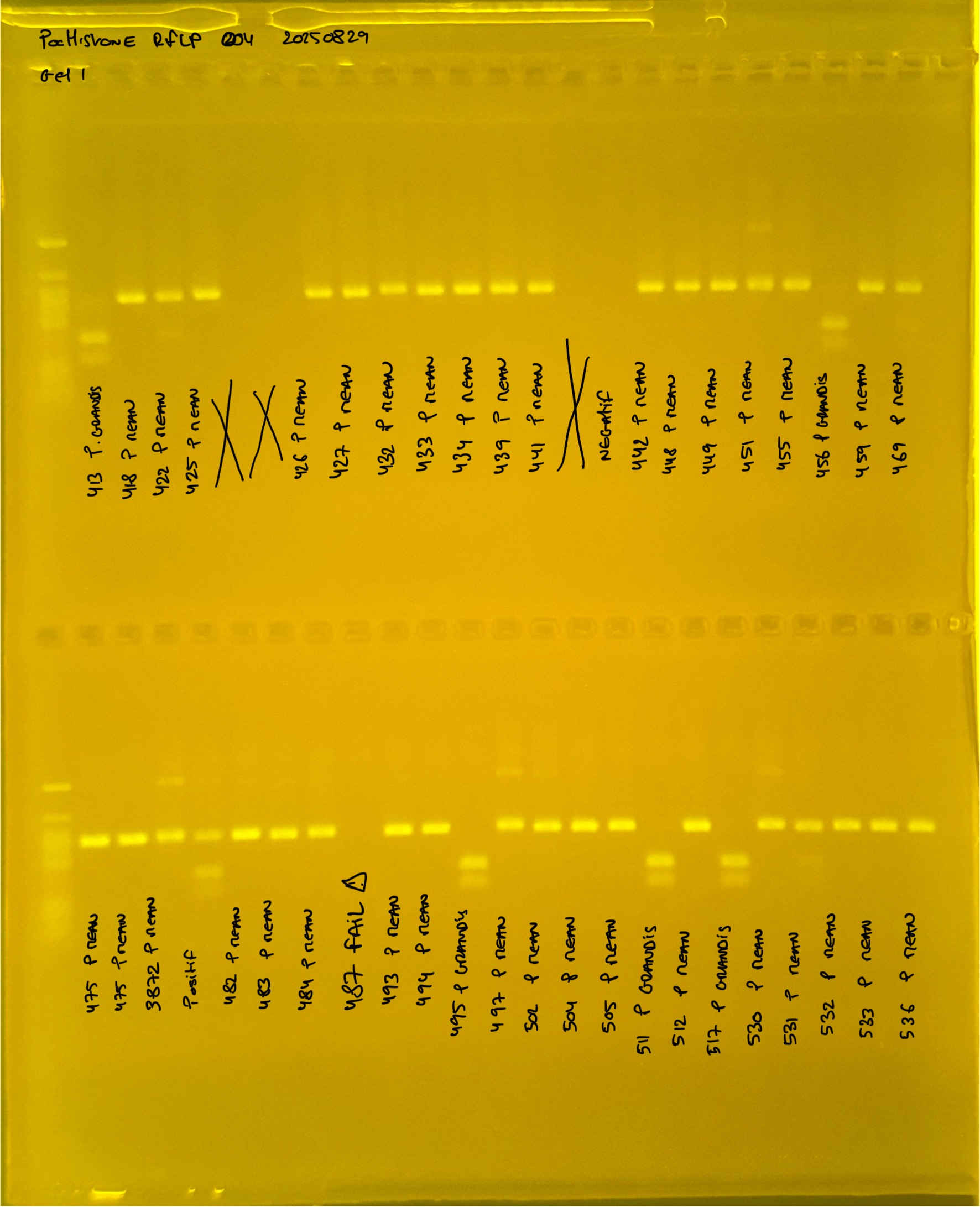
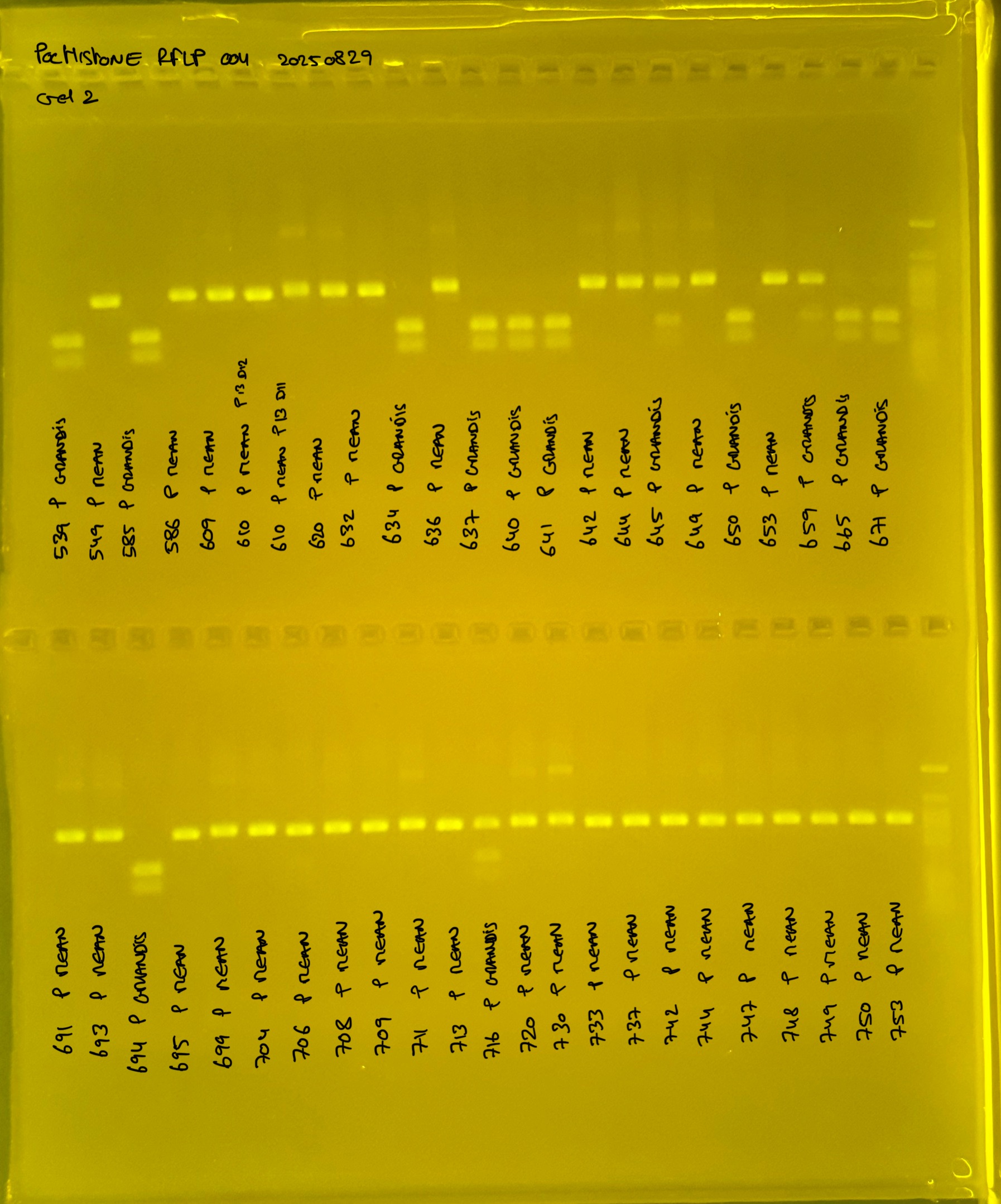
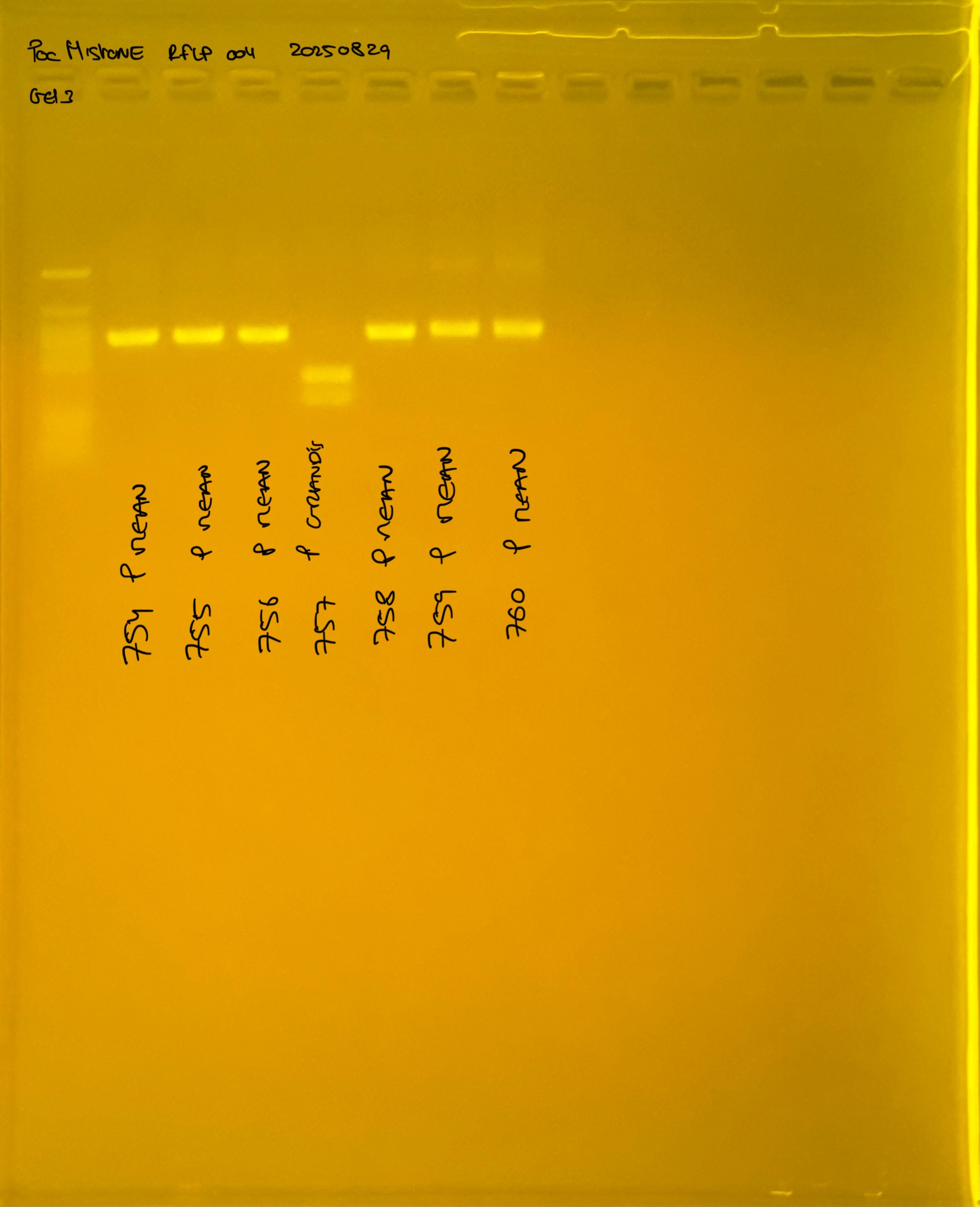
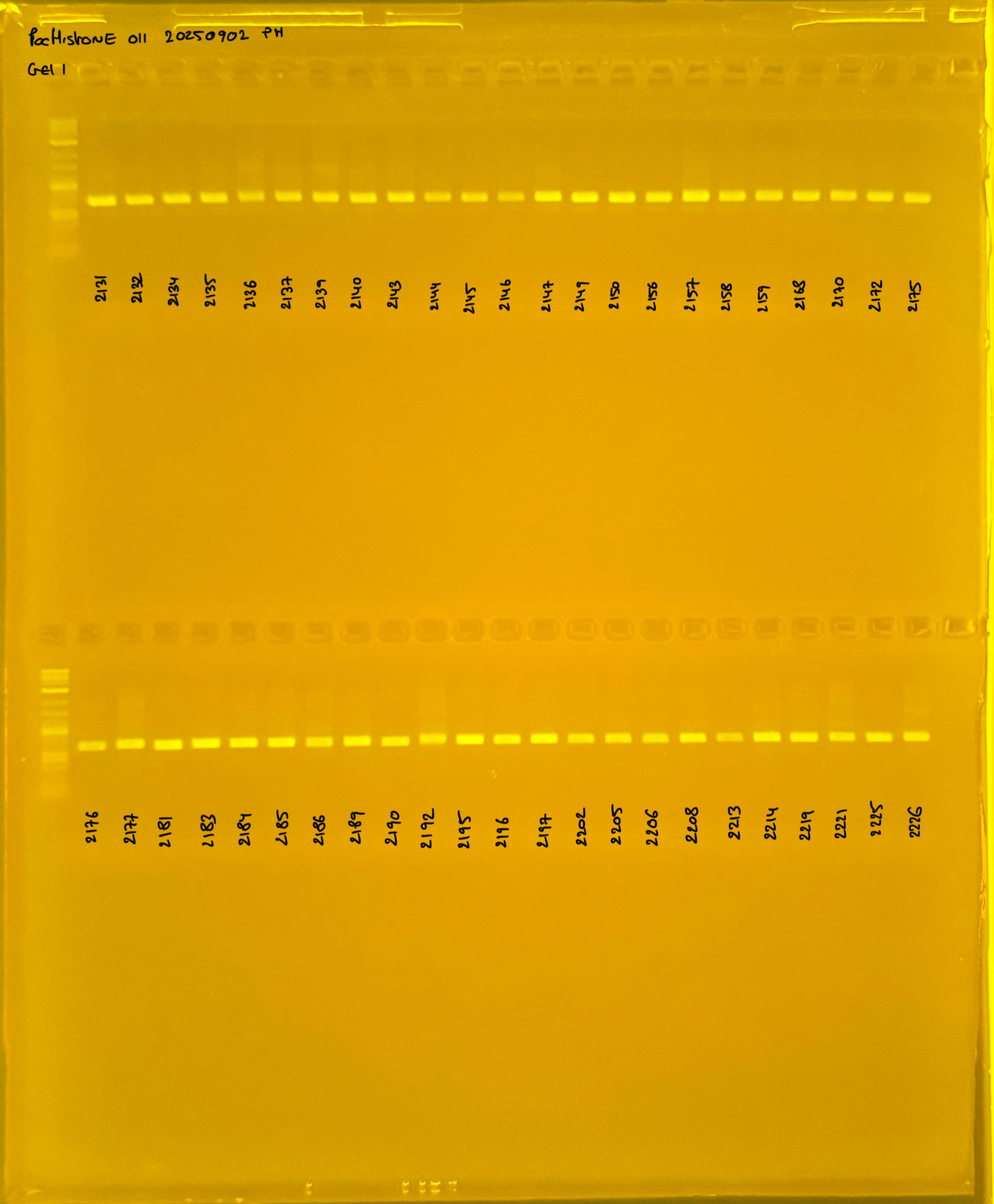
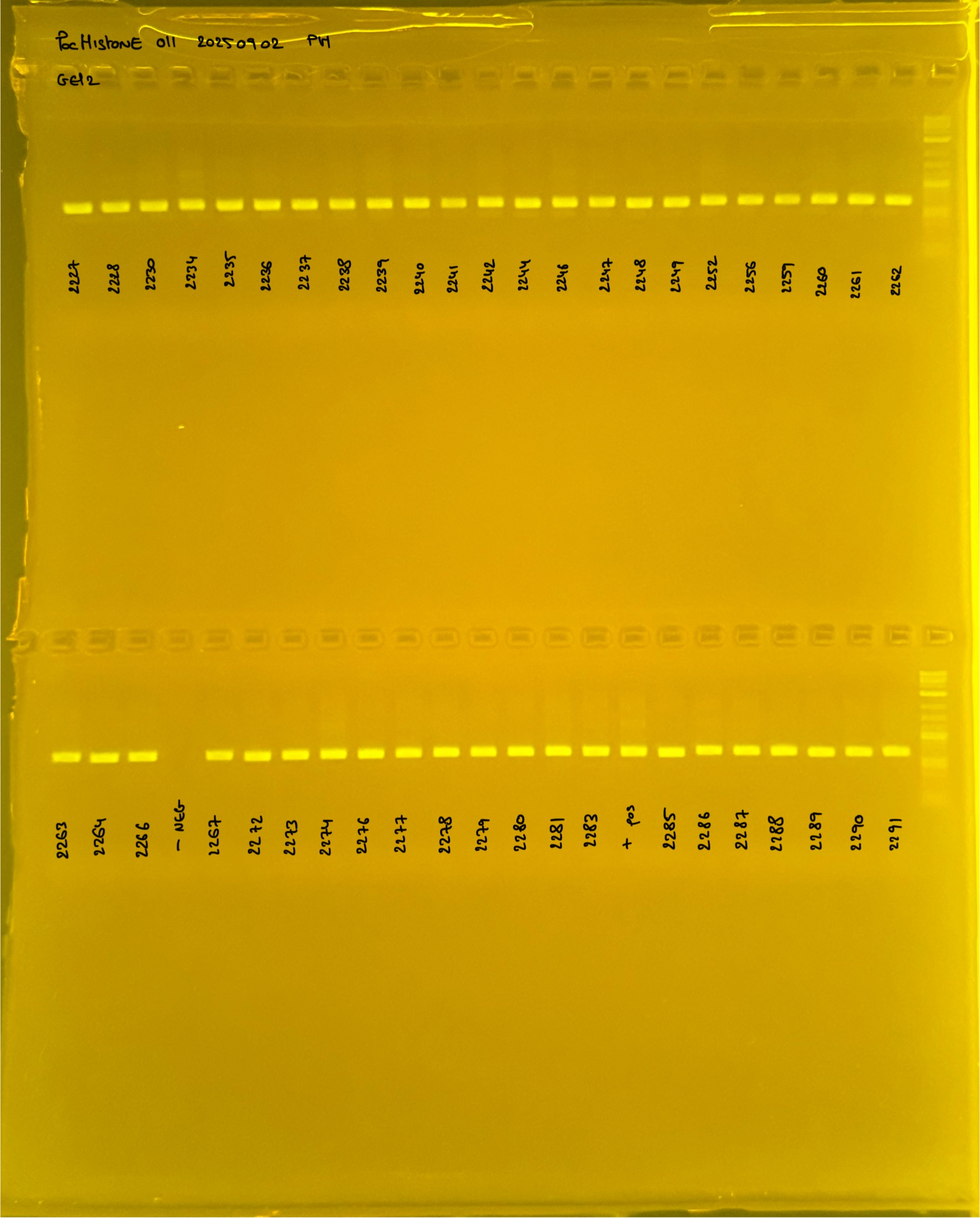
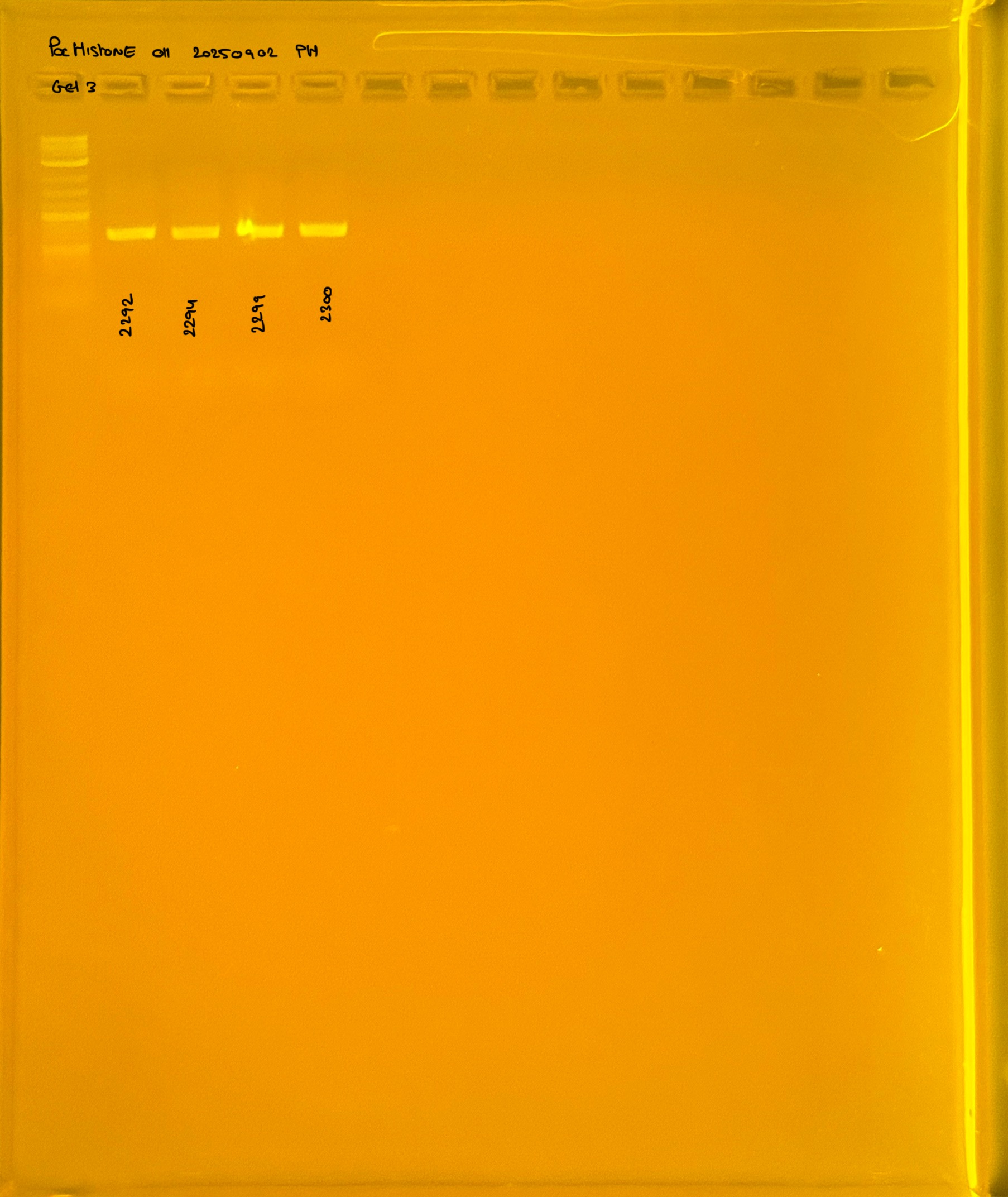
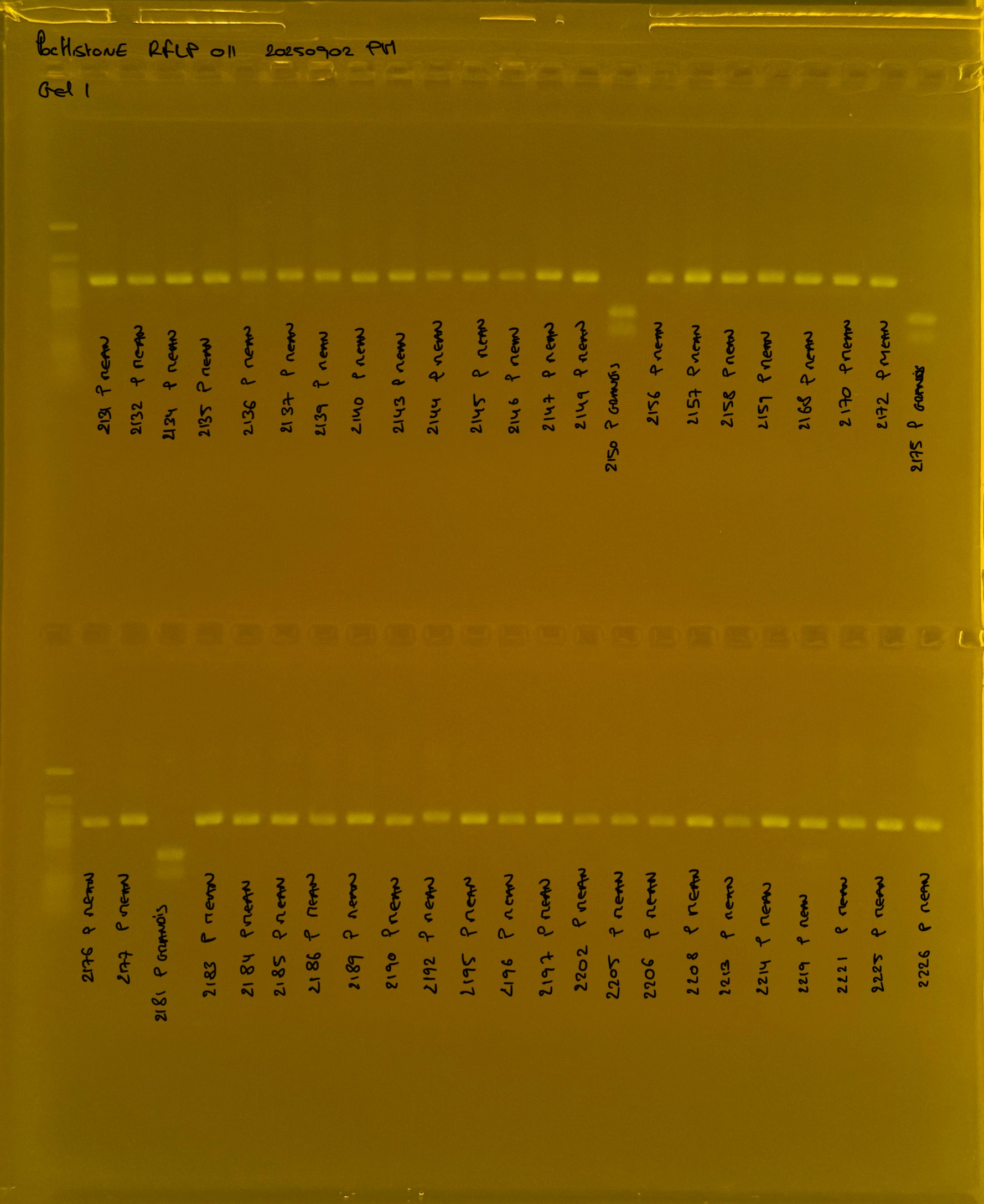
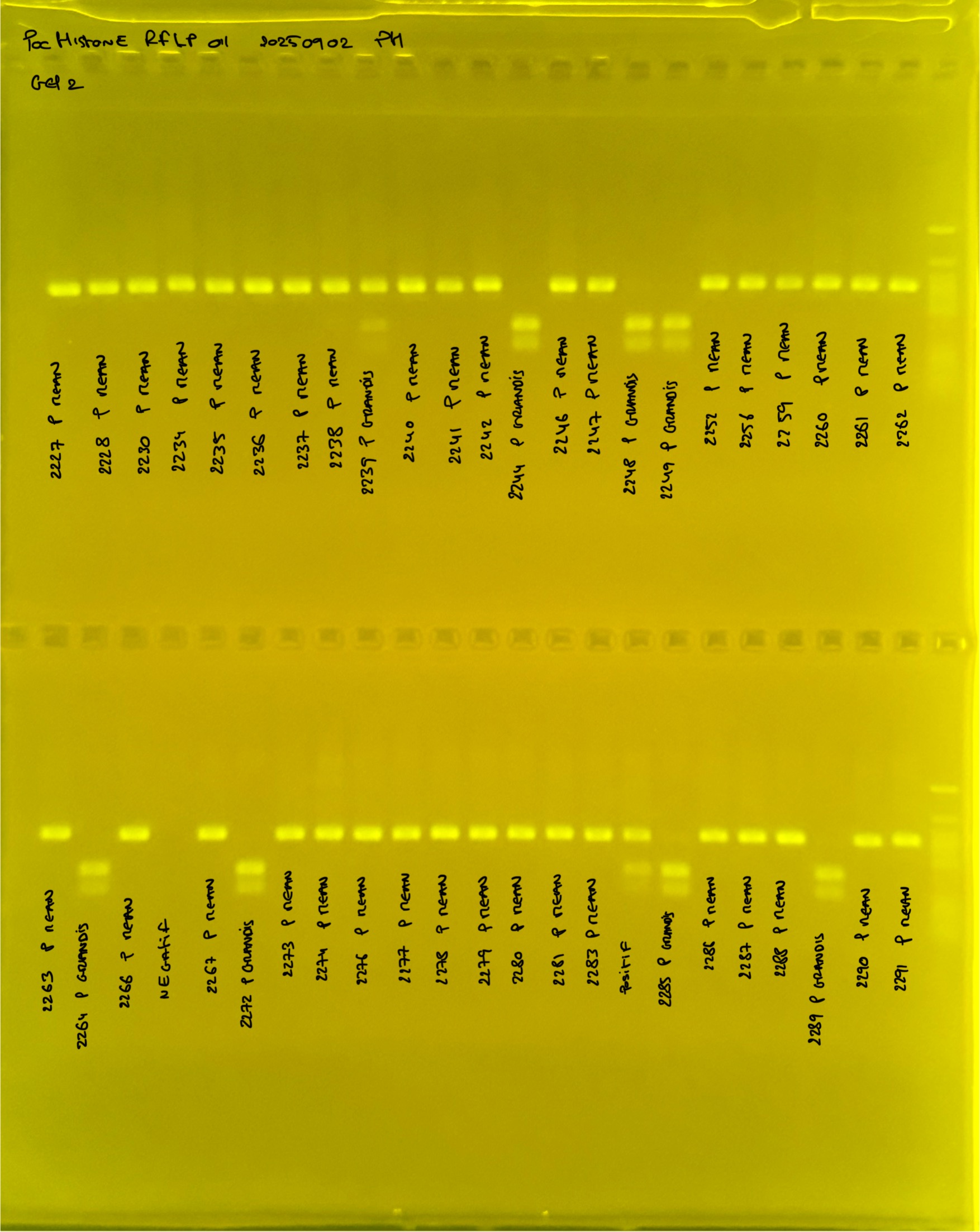
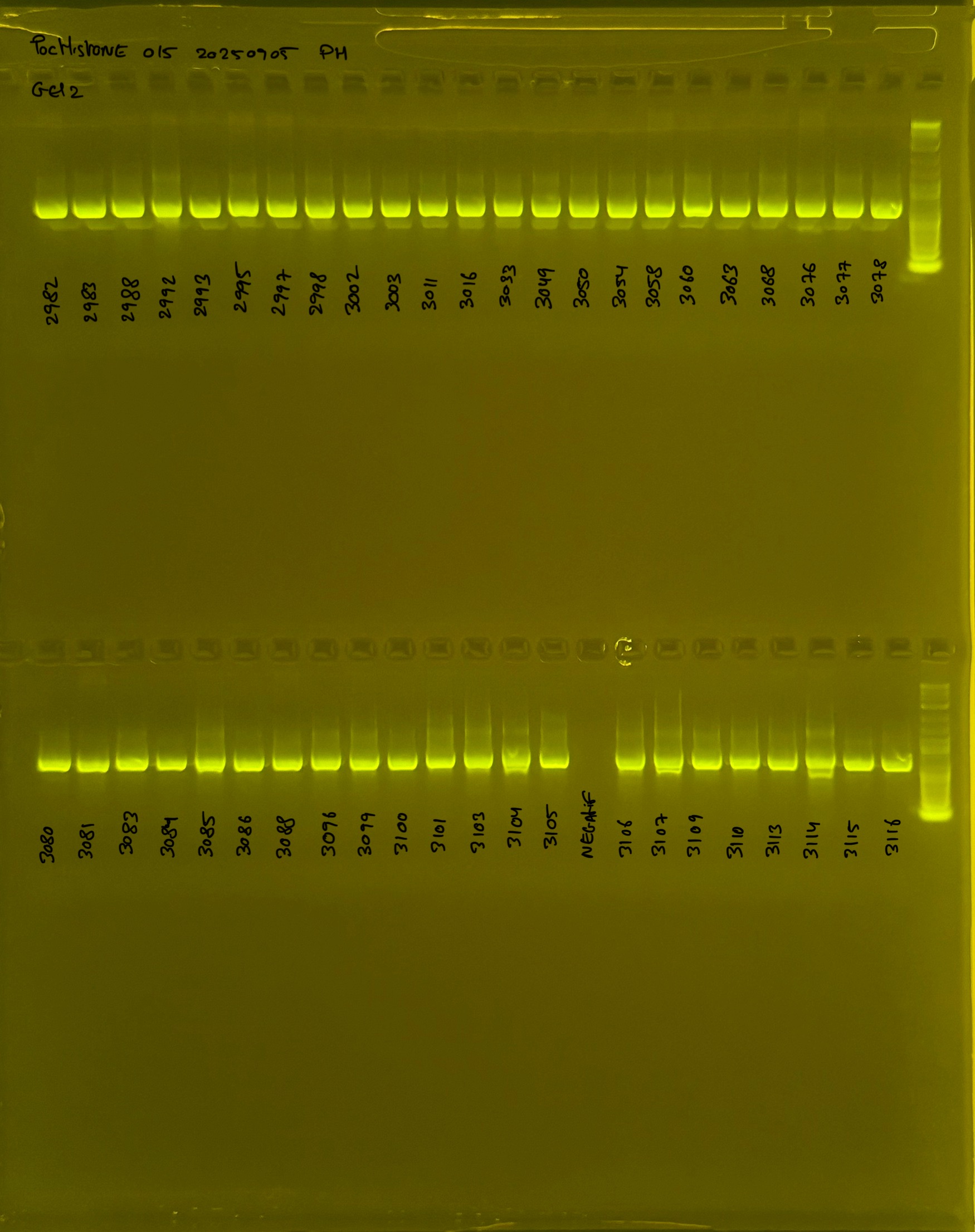
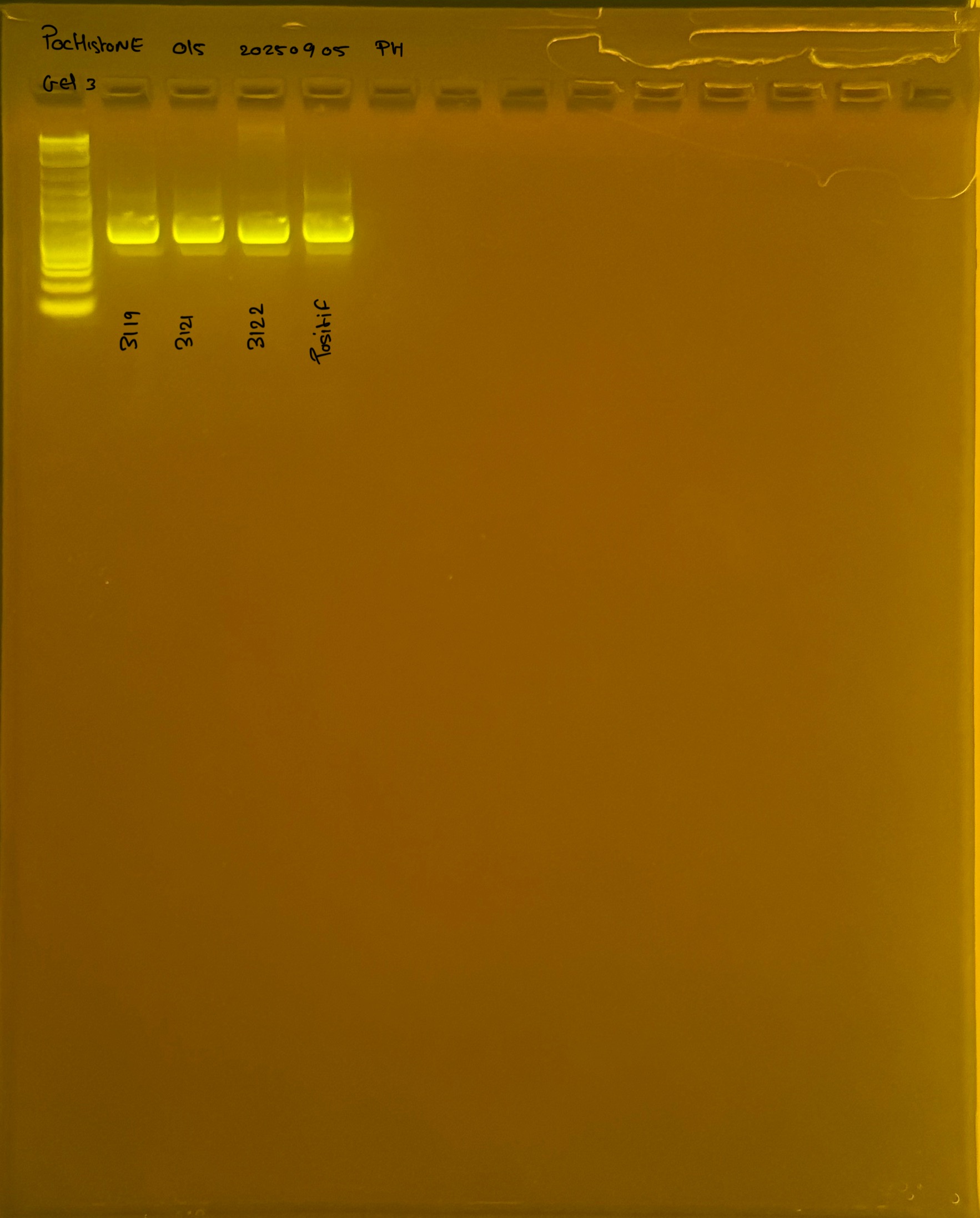
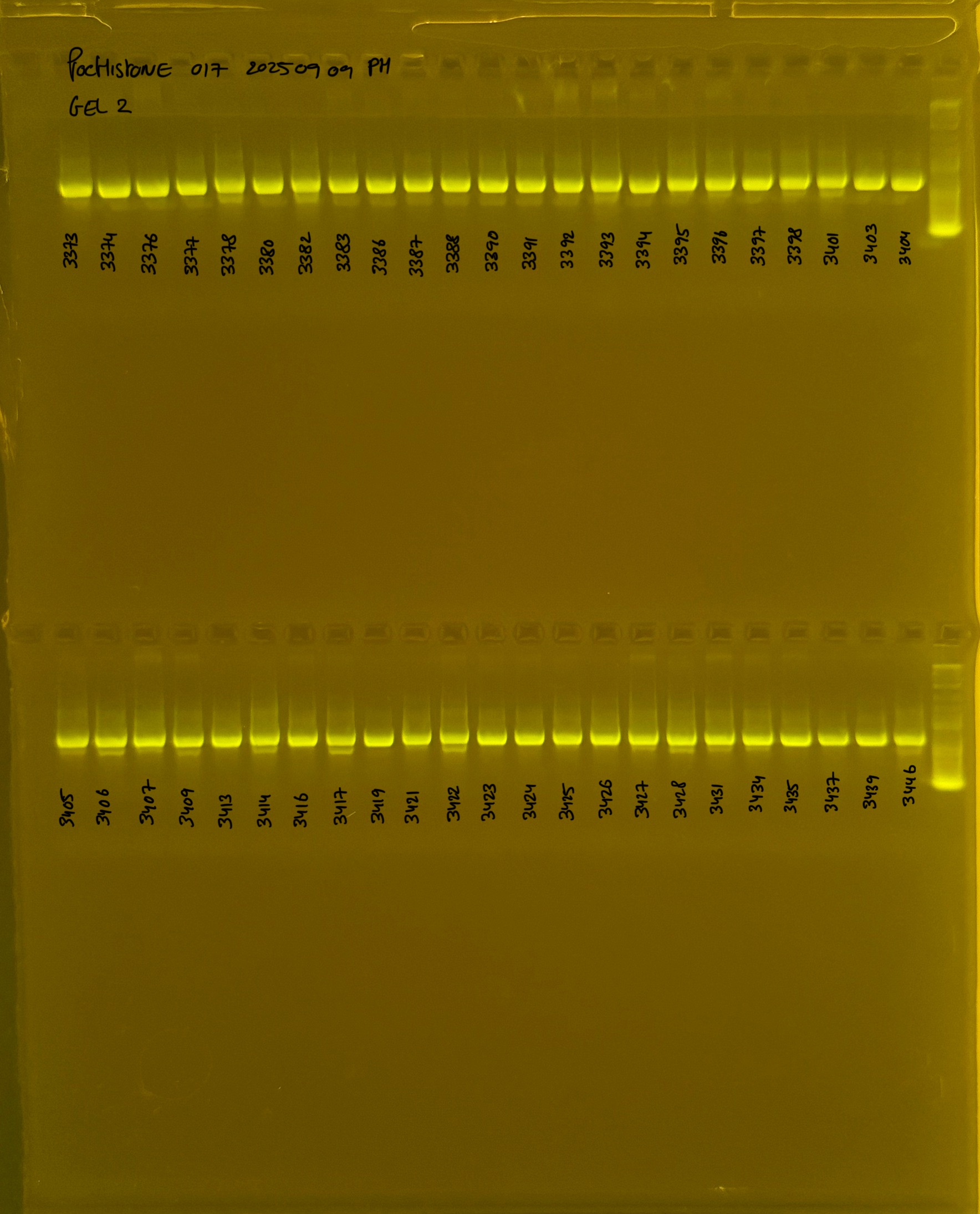
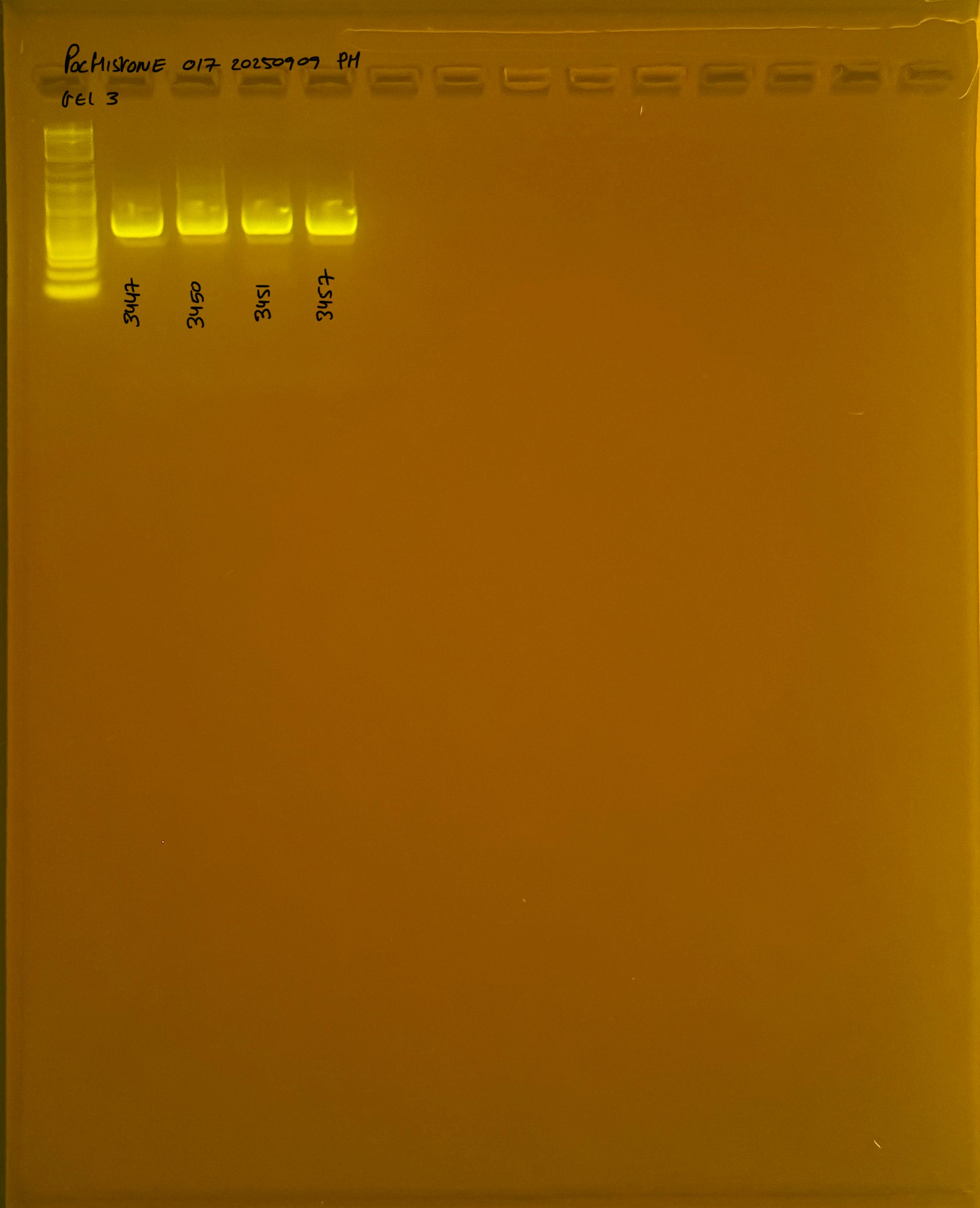
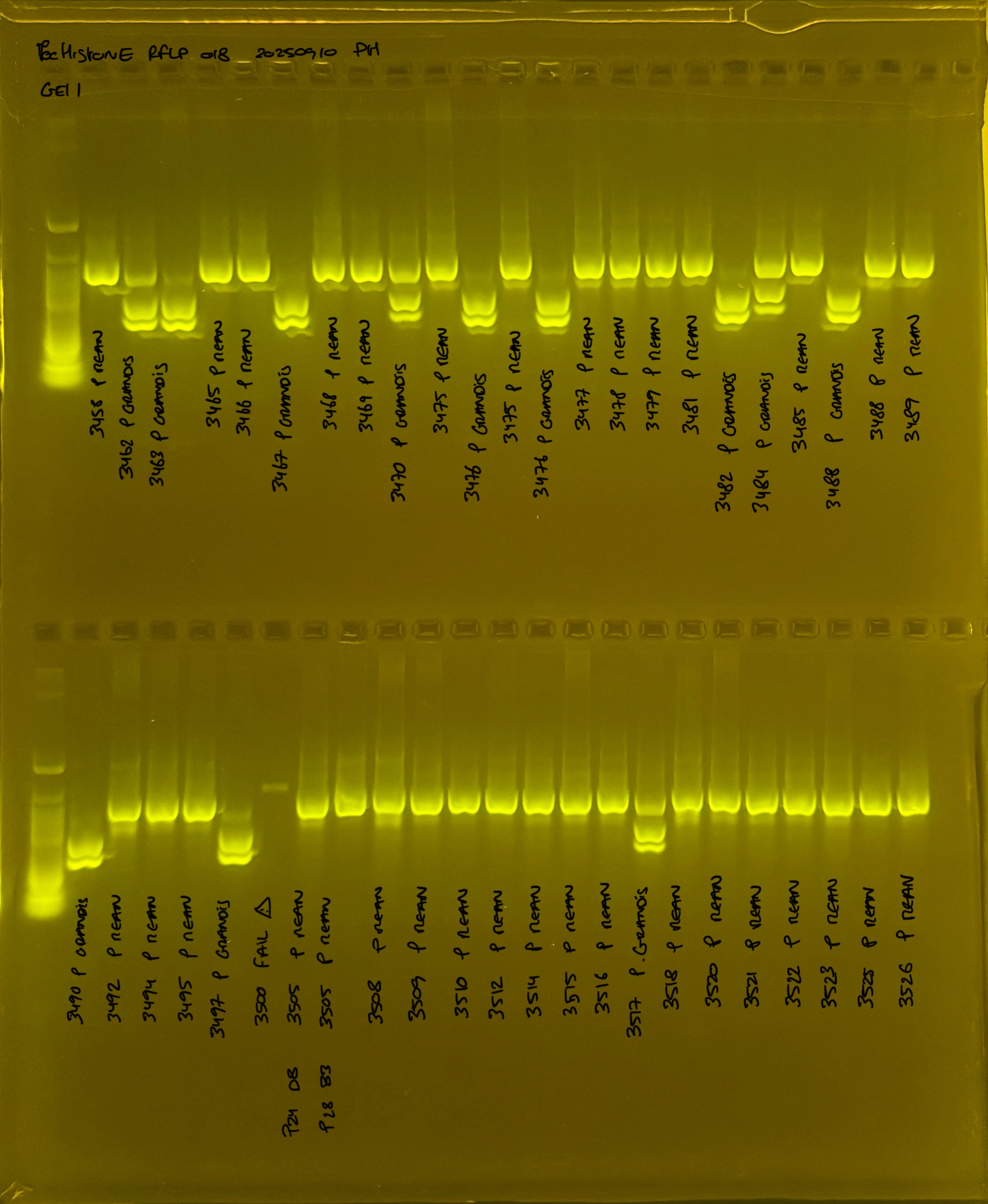
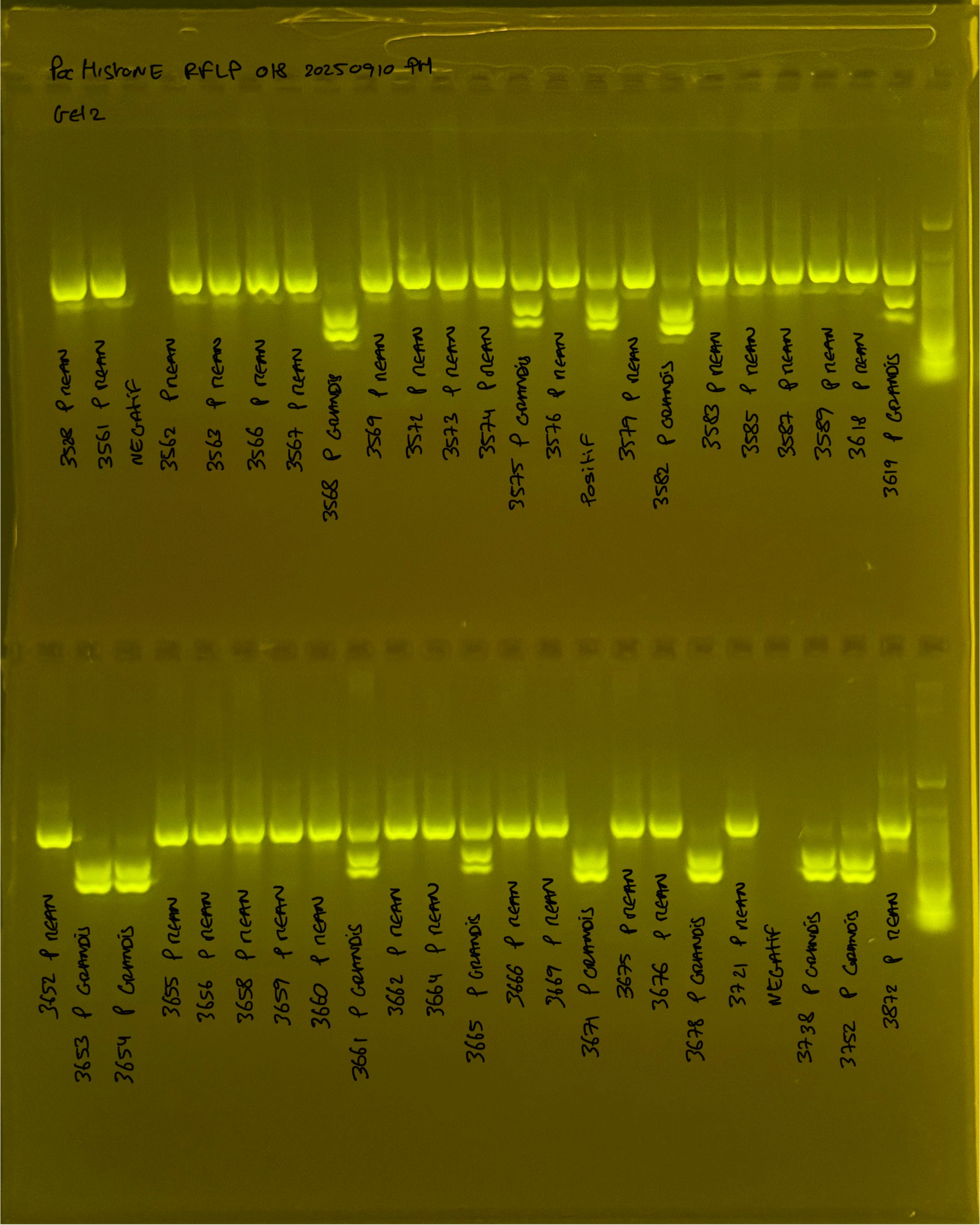
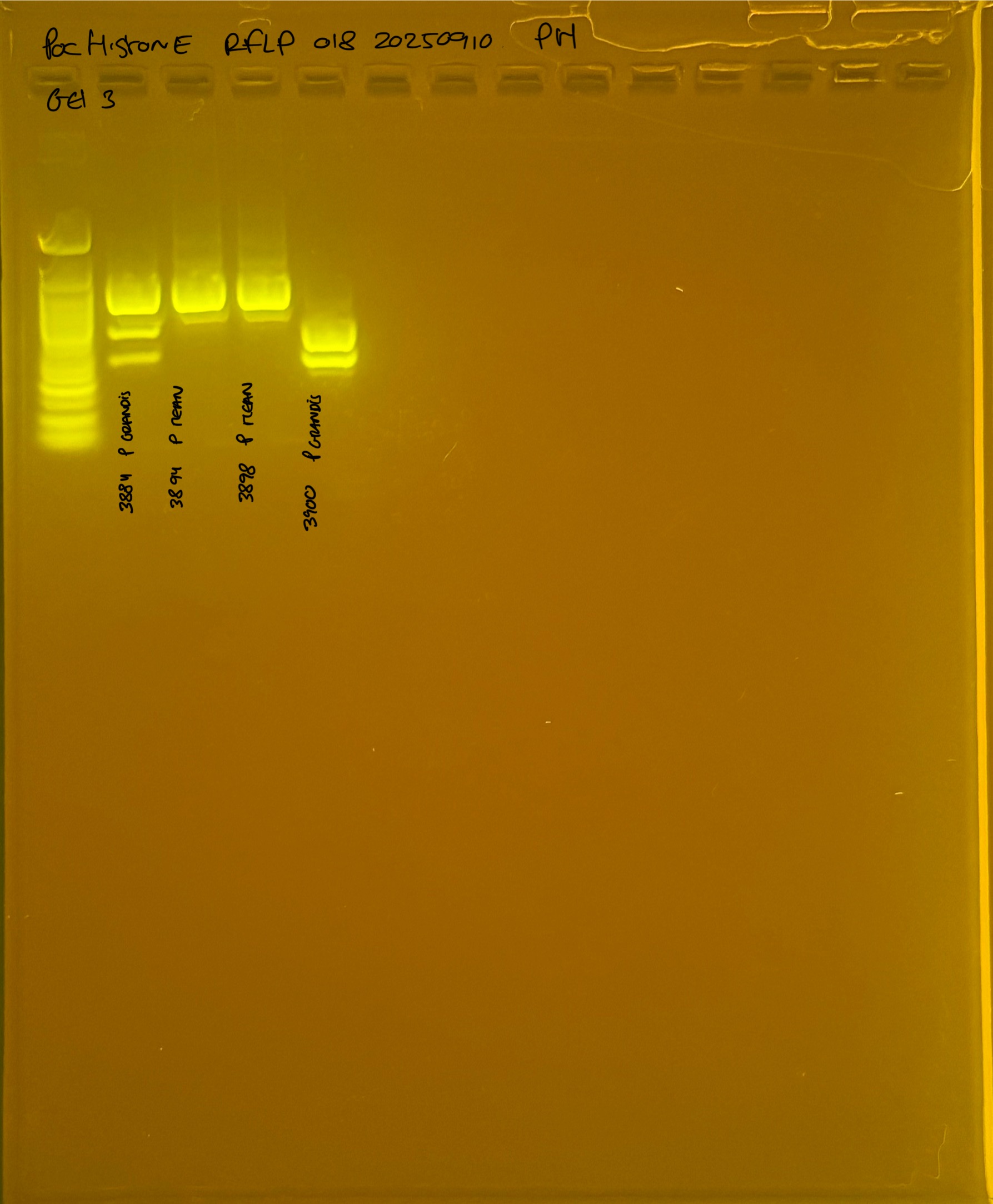
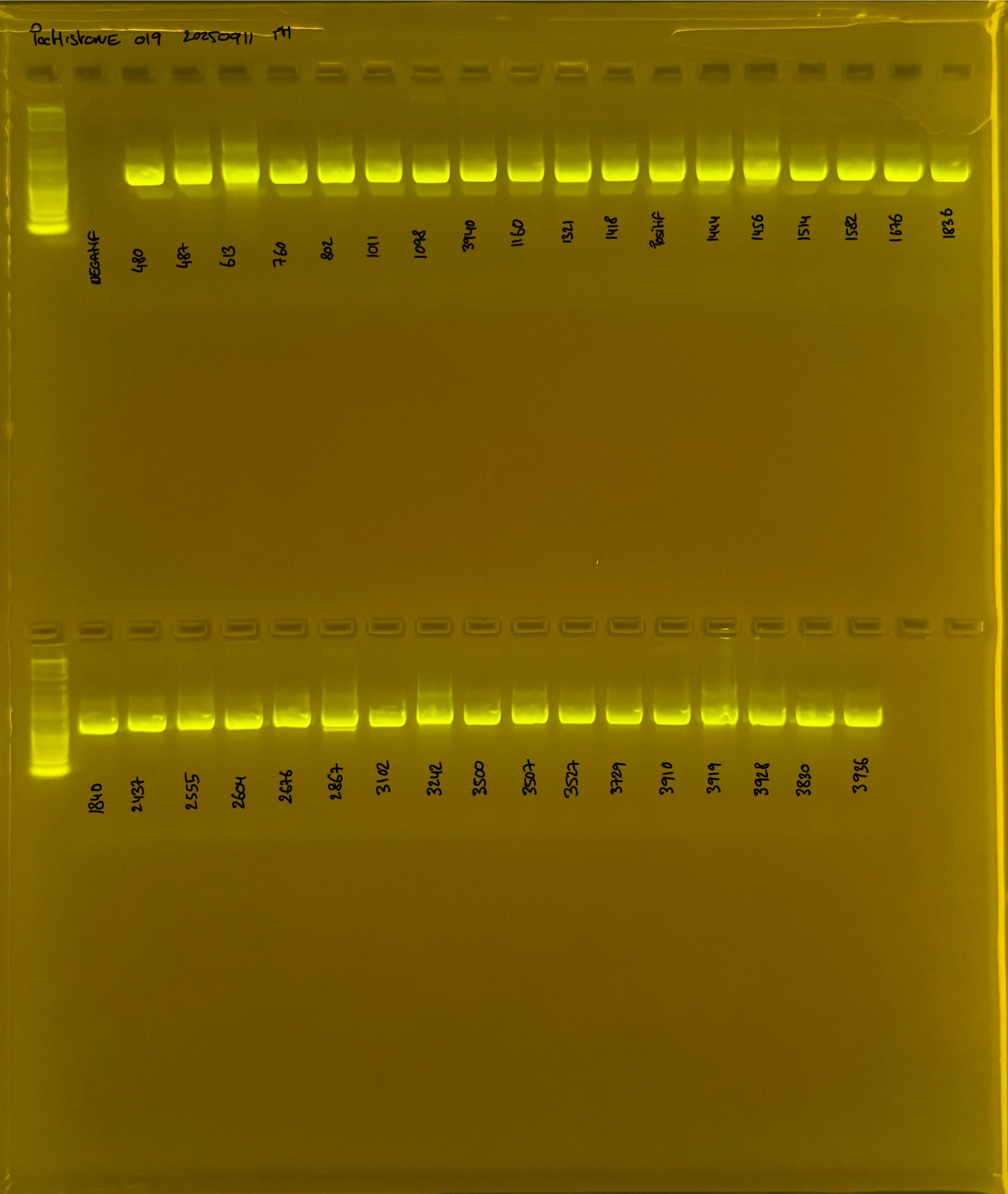
Gel pictures
20240411 plate002 gel 001 mtORF after extraction:
20240411 plate002 gel 002 mtORF after extraction:
20240411 plate002 gel 003 mtORF after extraction:
20250226 plate003 gel 001 mtORF after extraction:
20250226 plate003 gel 003 mtORF after extraction:
20250226 plate004 gel 001 mtORF after extraction:
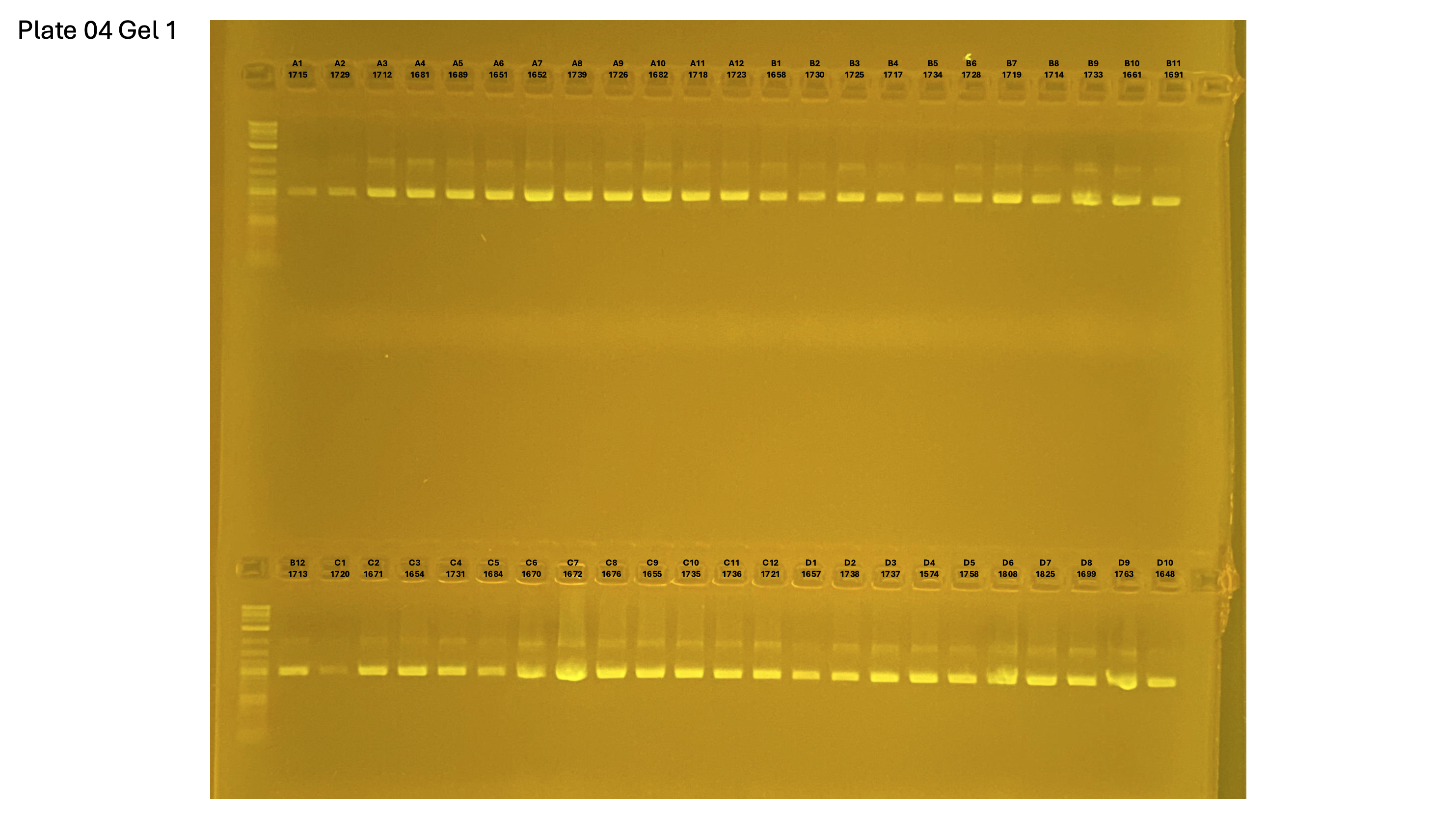
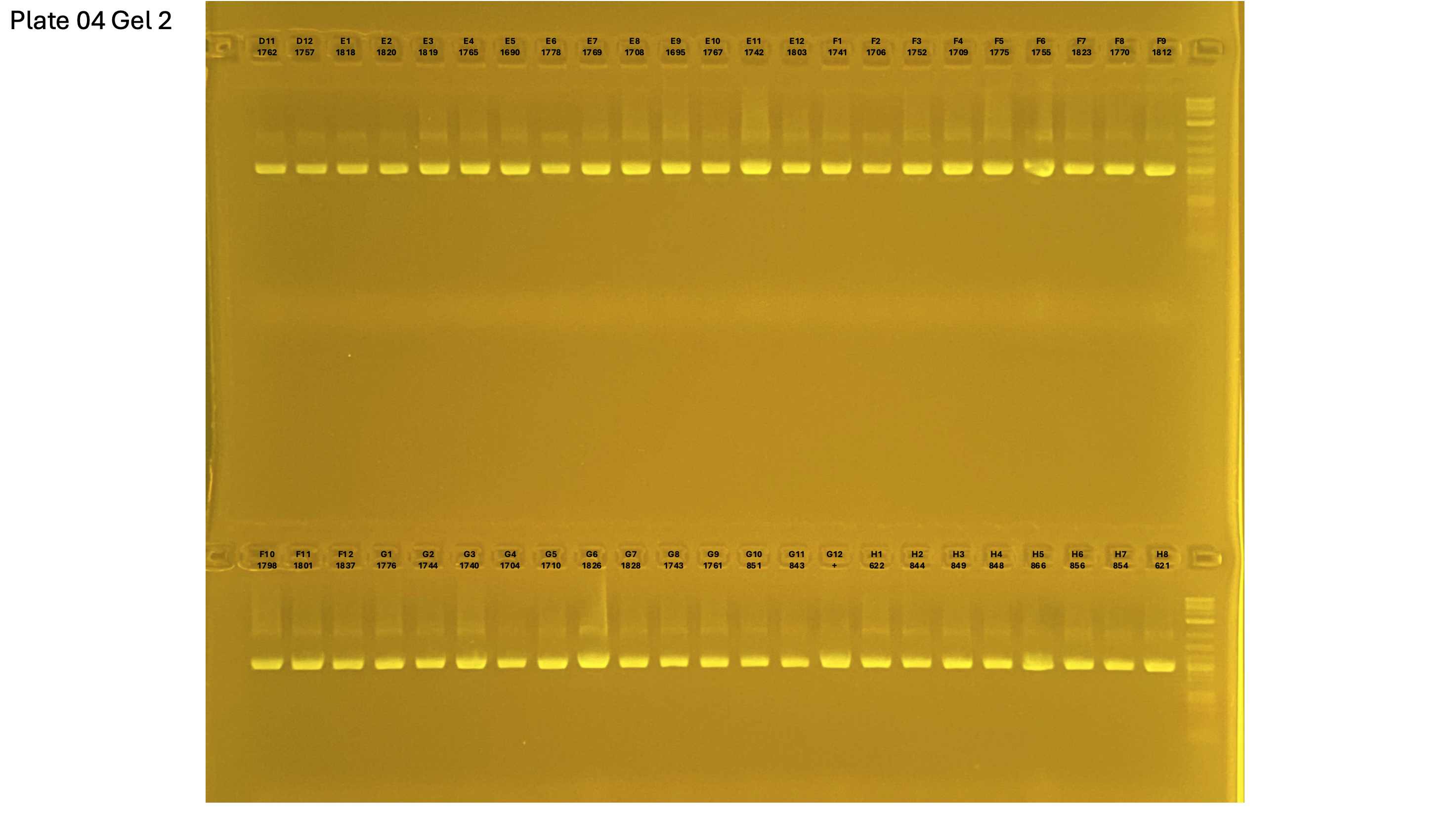
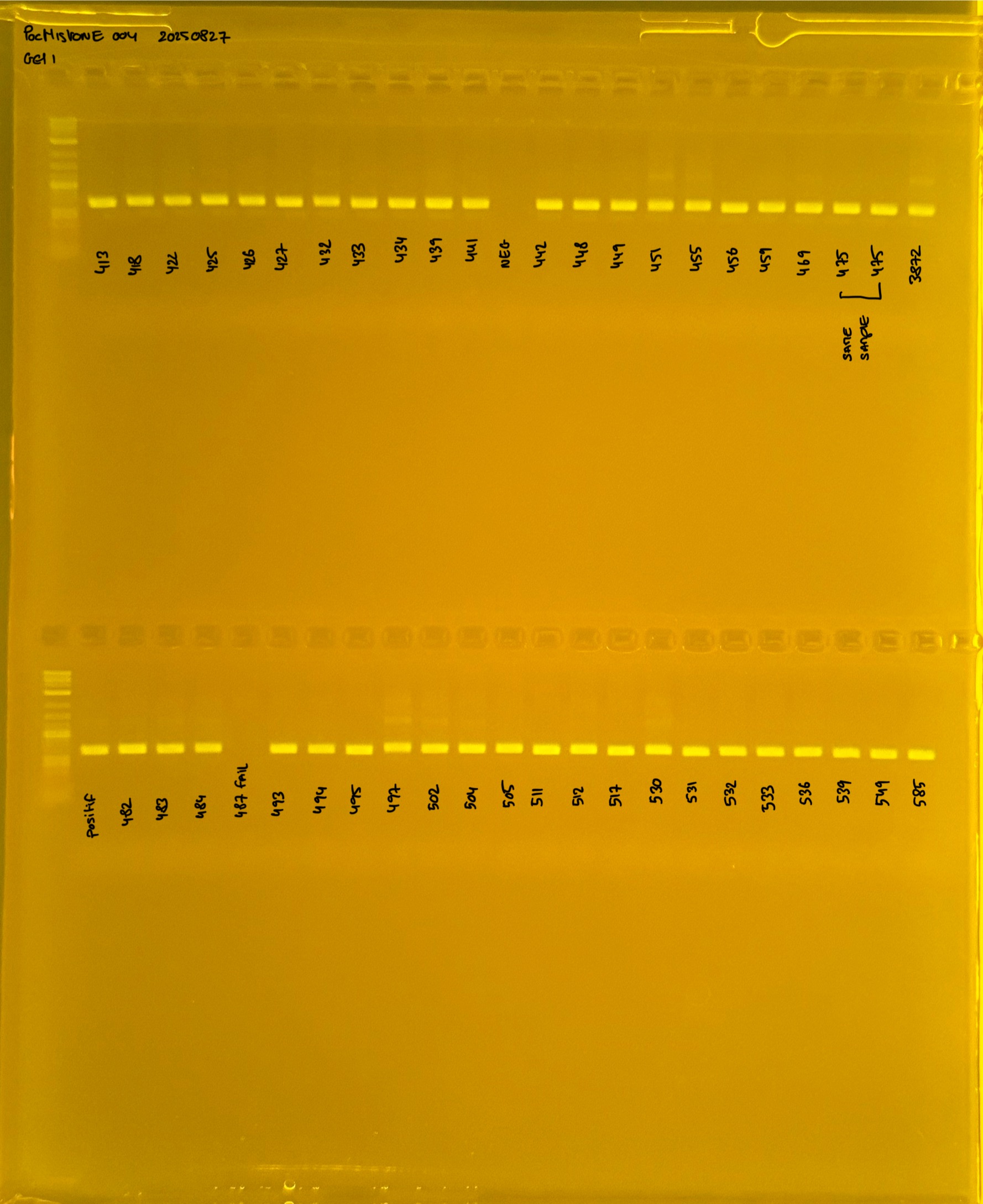
20250226 plate004 gel 002 mtORF after extraction:
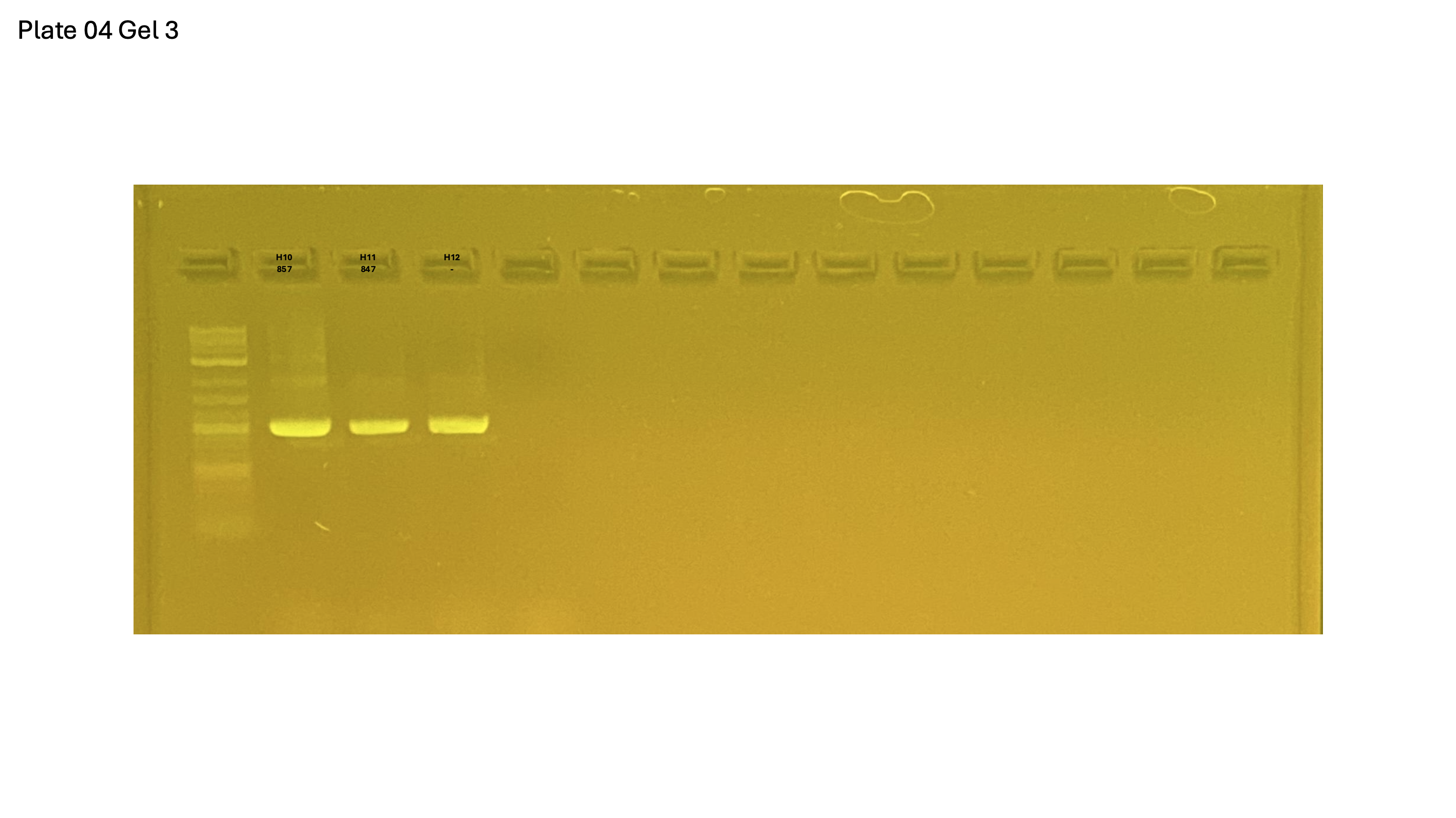
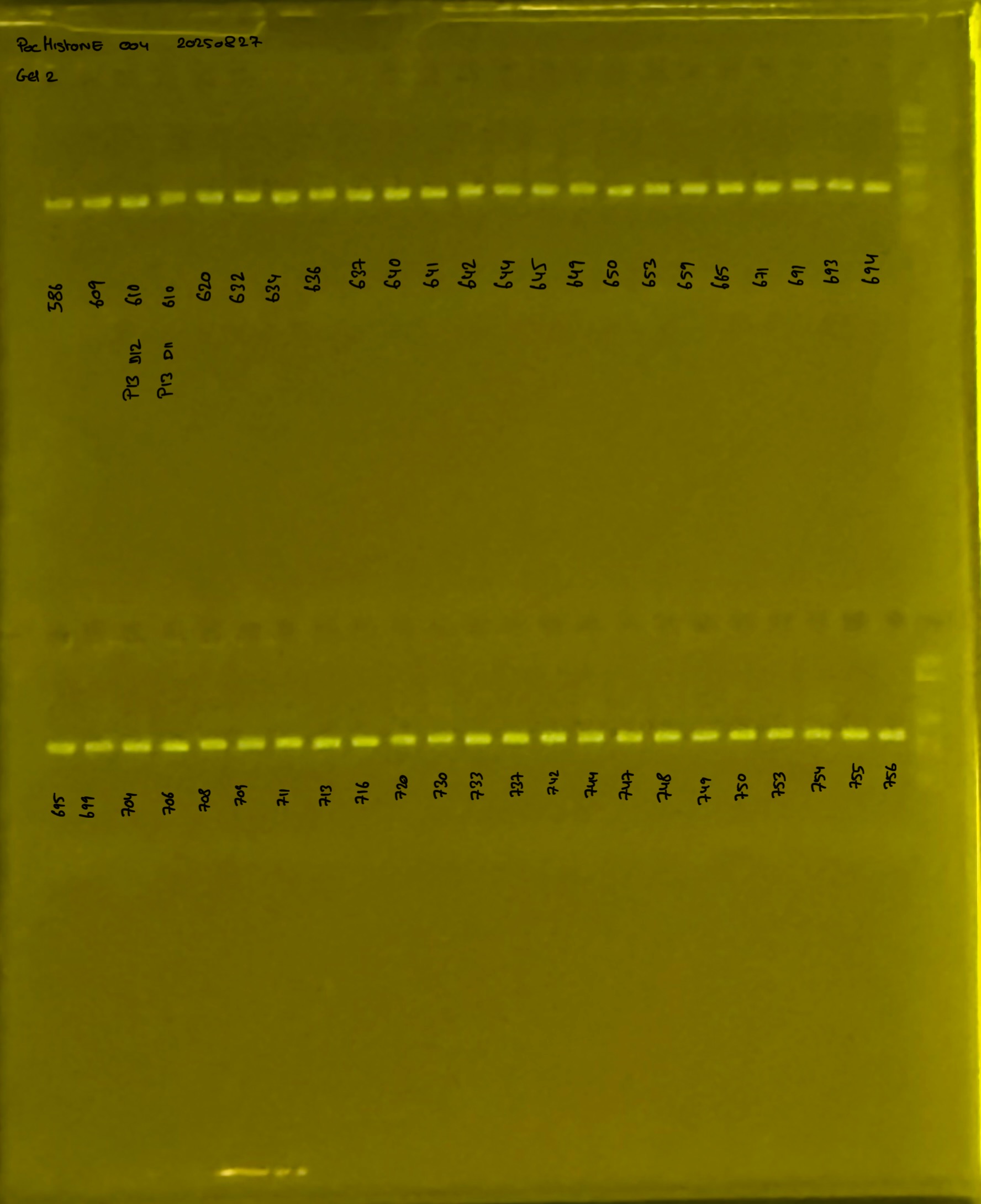
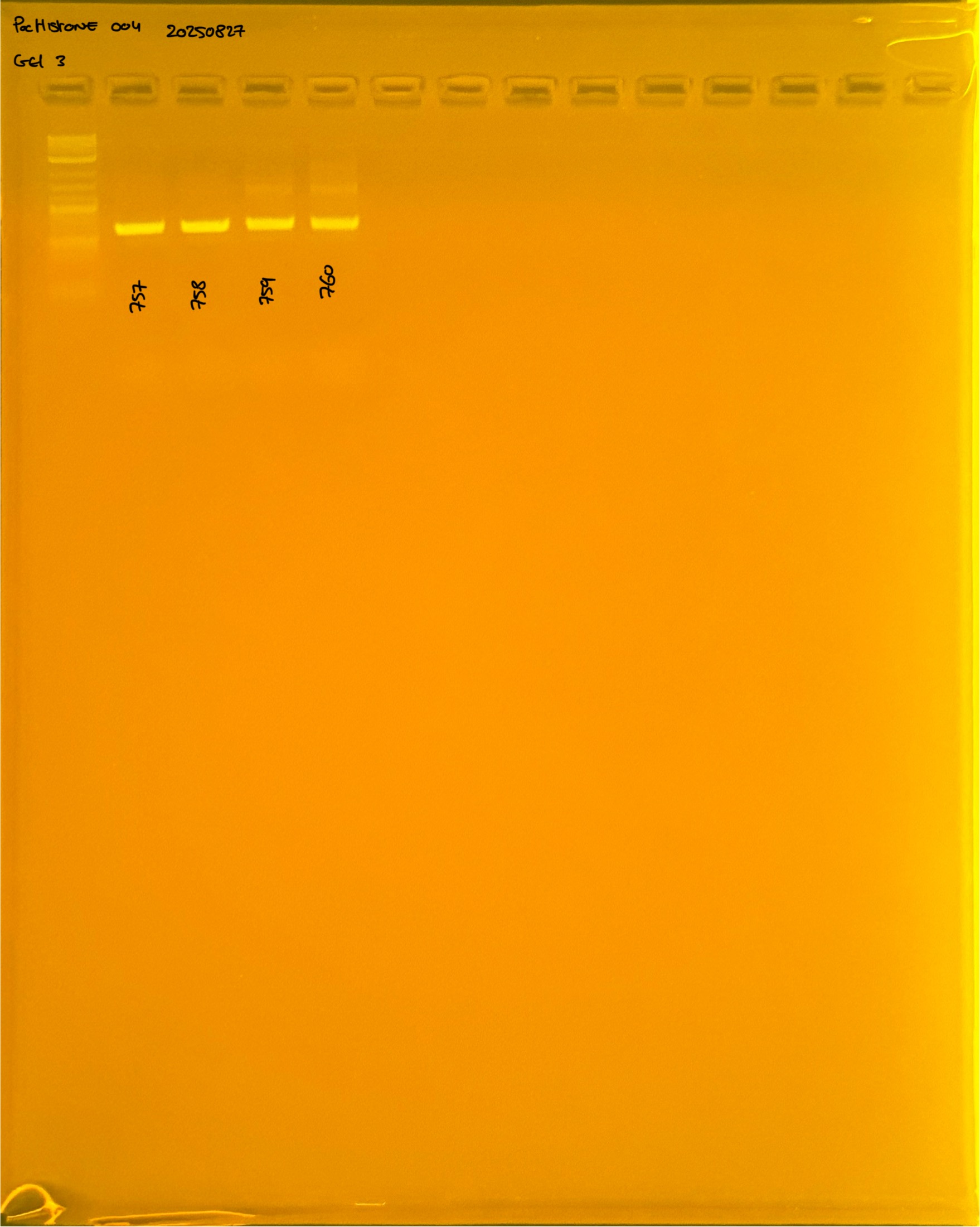
20250226 plate004 gel 003 mtORF after extraction:
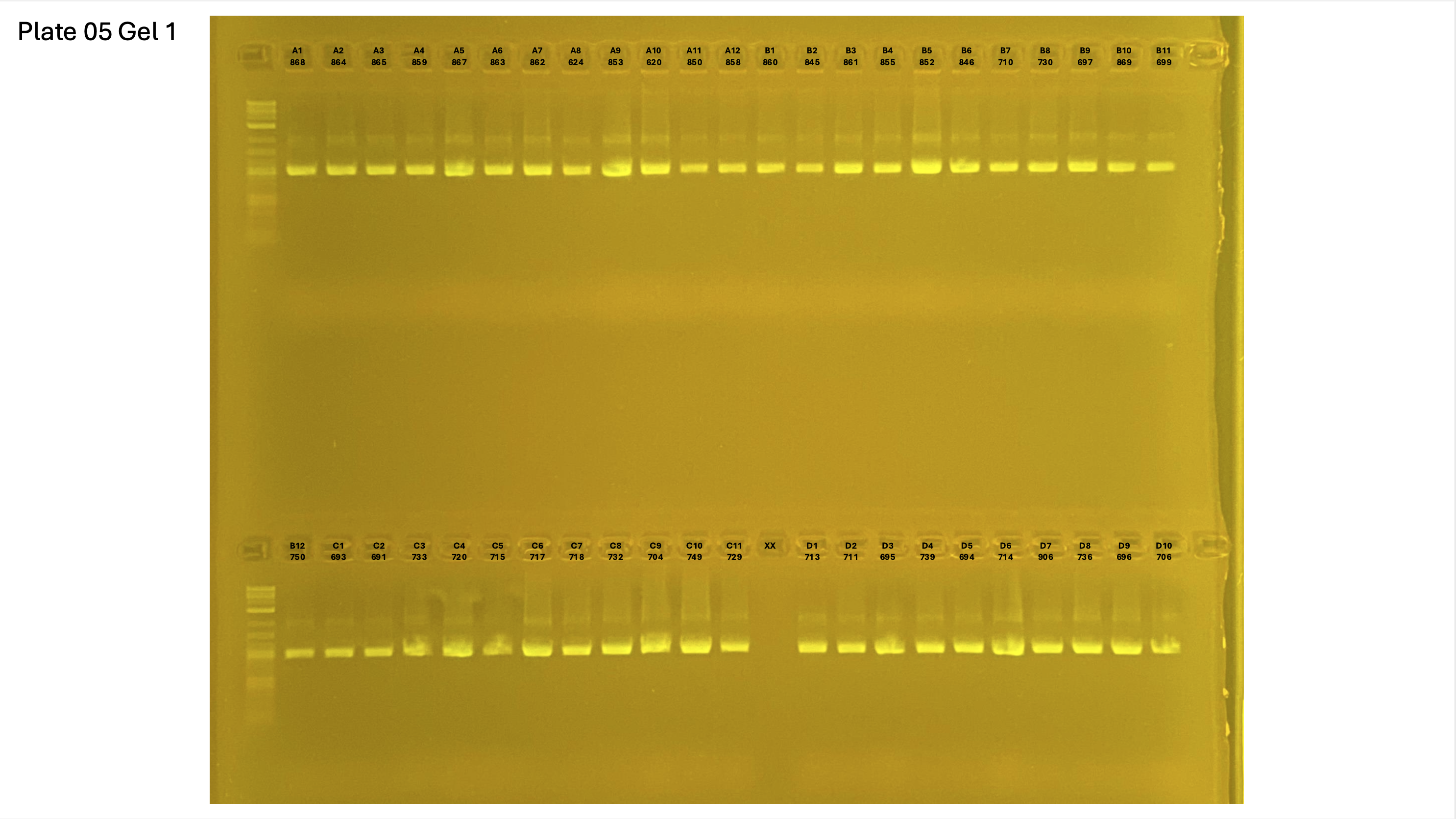
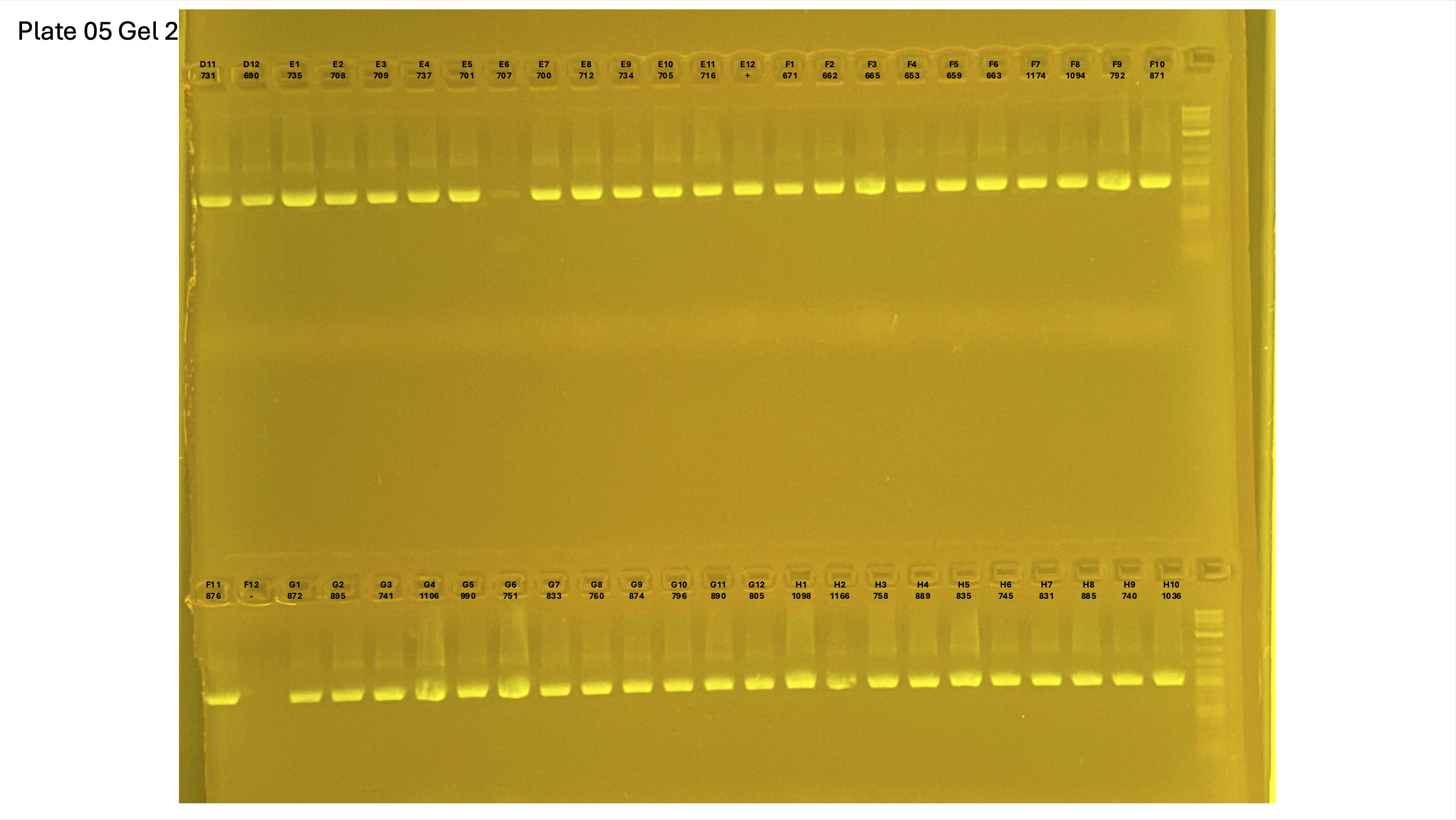
20250226 plate005 gel 001 mtORF after extraction:
20250226 plate005 gel 002 mtORF after extraction:
20250226 plate005 gel 003 mtORF after extraction:
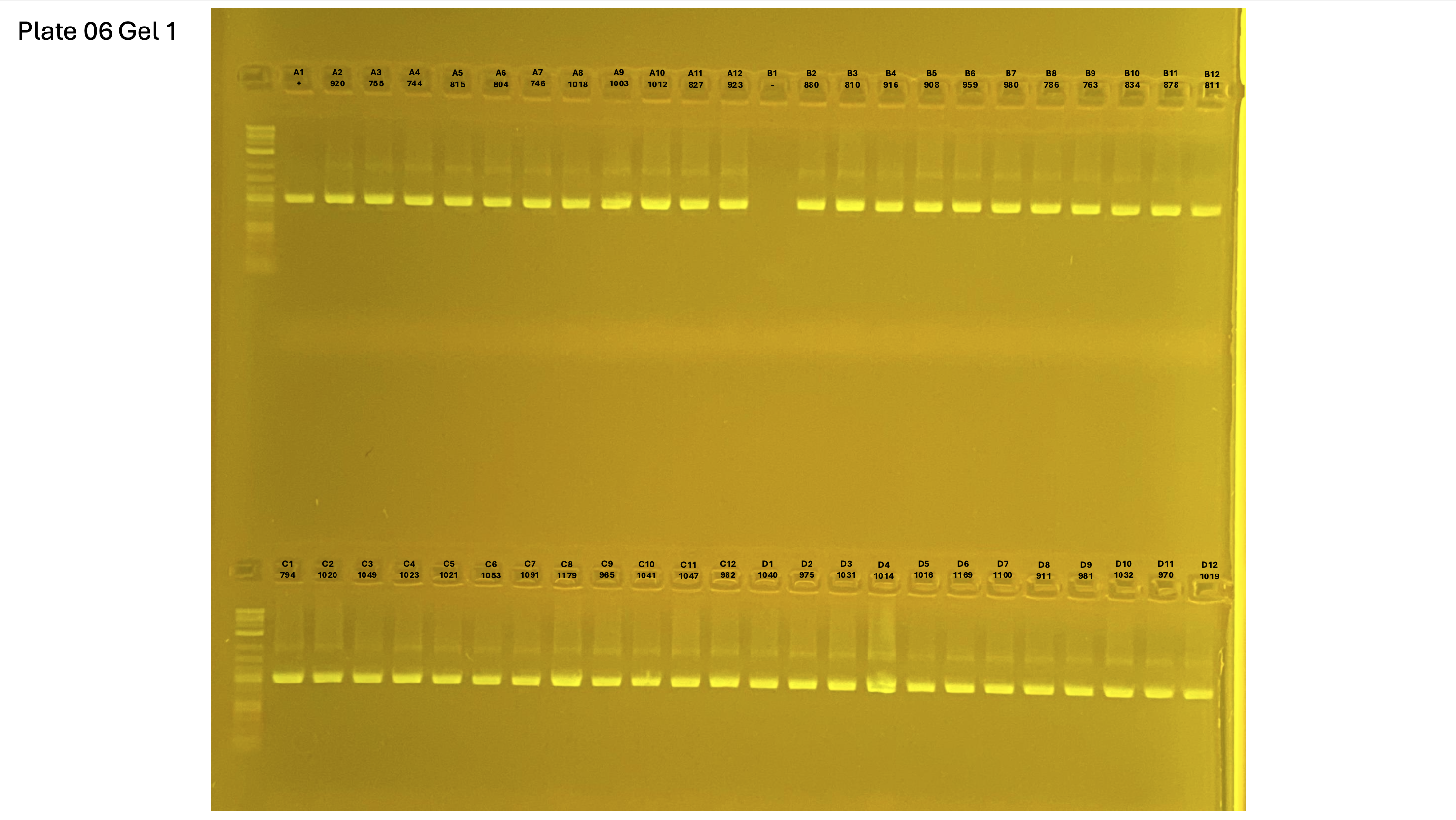
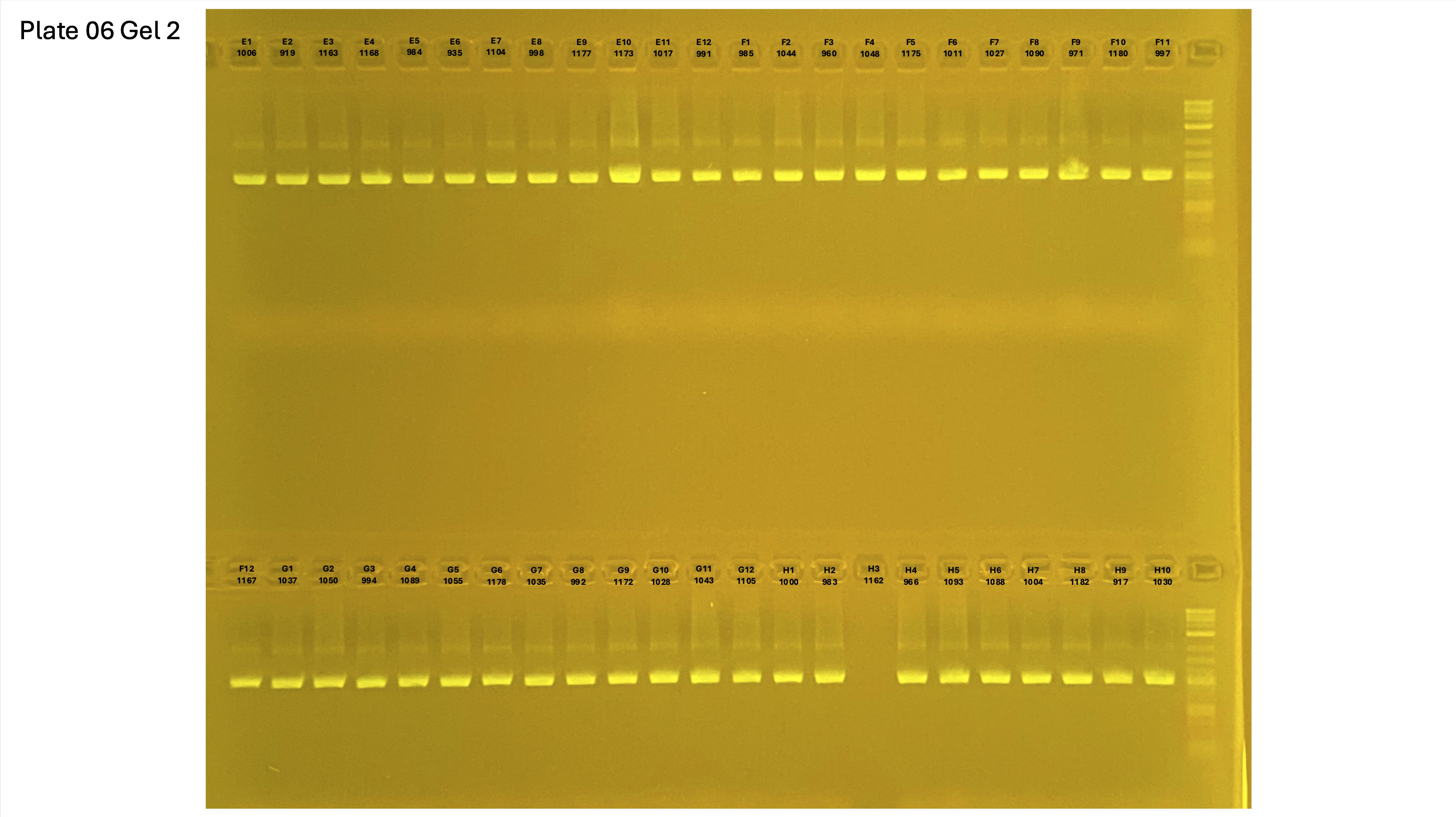
20250226 plate006 gel 001 mtORF after extraction:
20250226 plate006 gel 002 mtORF after extraction:
20250226 plate006 gel 003 mtORF after extraction:
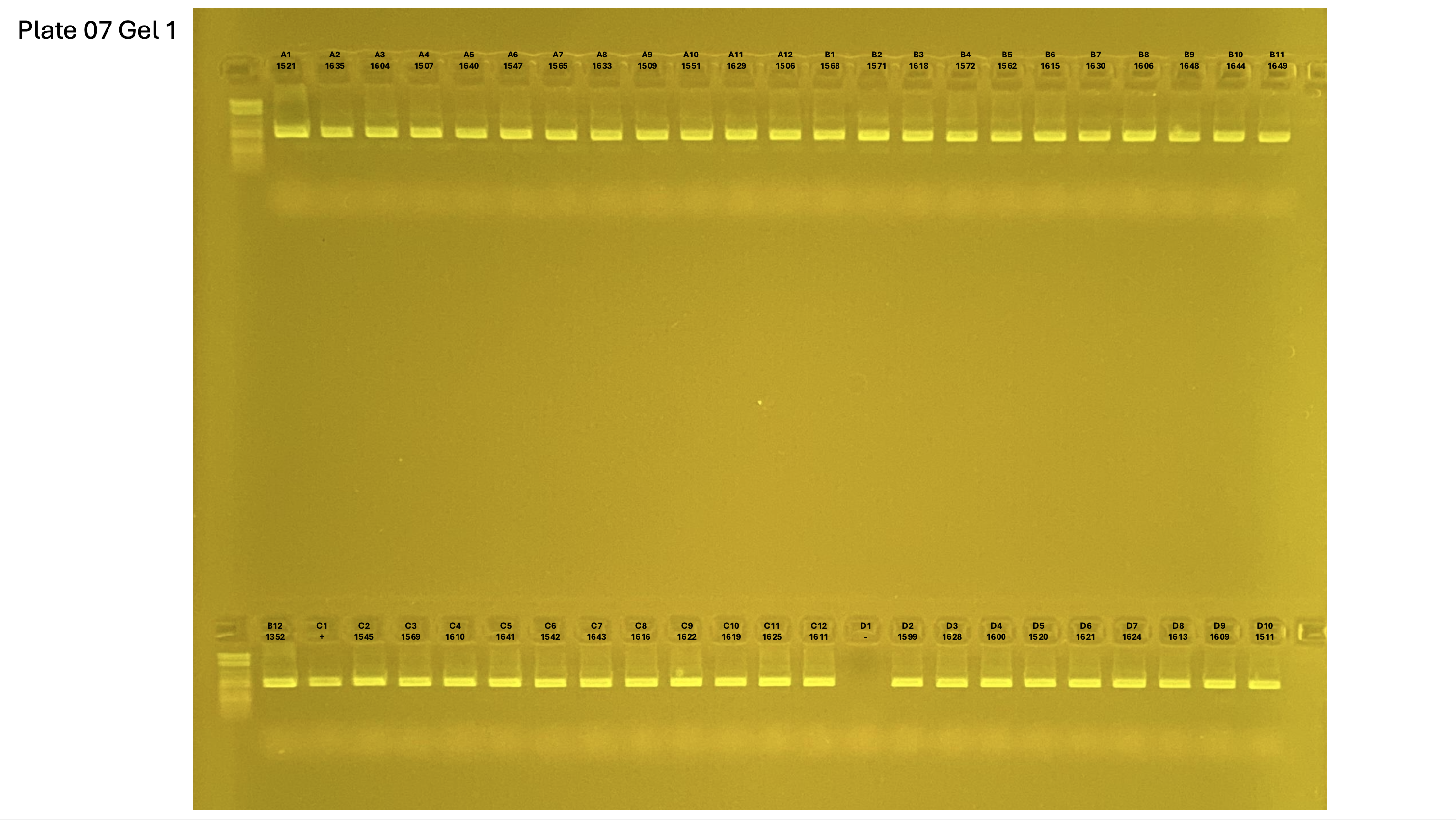
20250226 plate007 gel 001 mtORF after extraction:
20250226 plate007 gel 002 mtORF after extraction:
20250226 plate007 gel 003 mtORF after extraction:
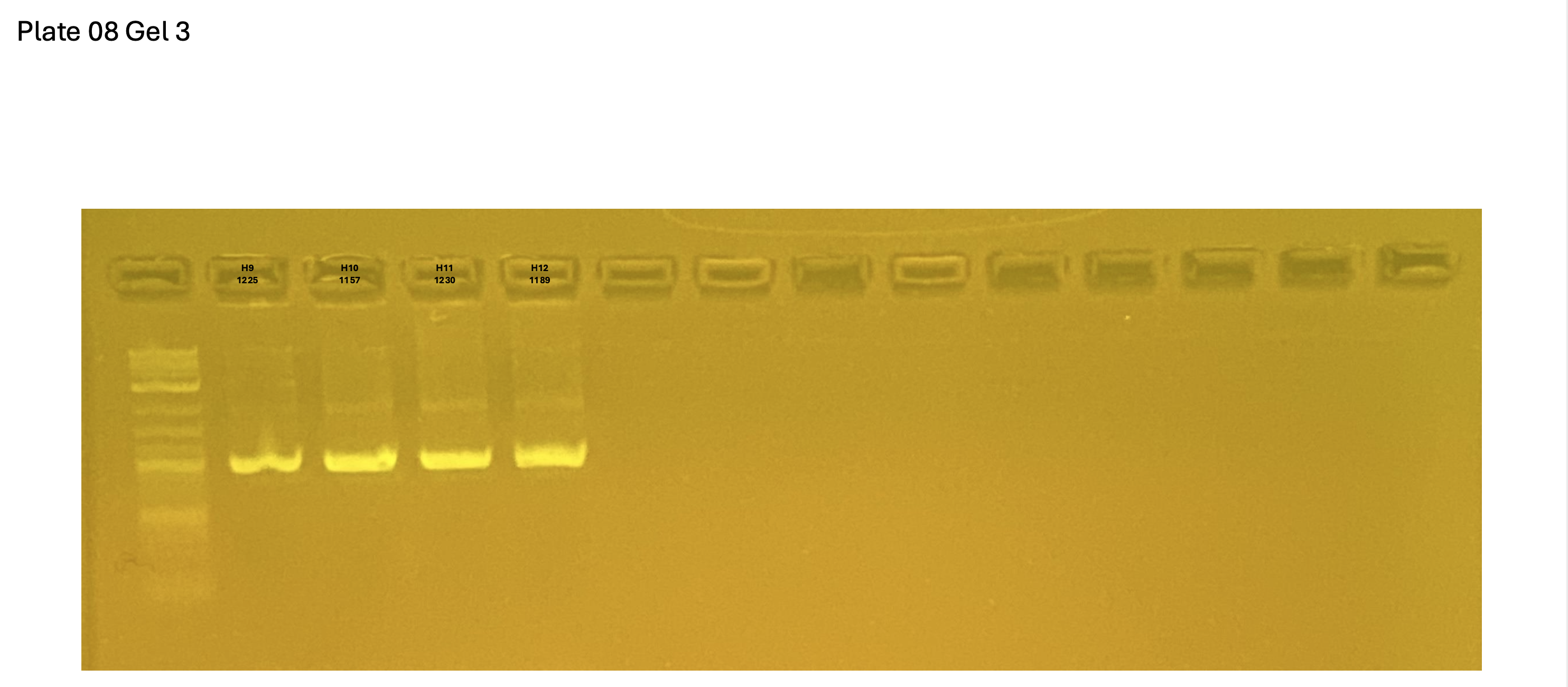
20250227 plate008 gel 001 mtORF after extraction:
20250227 plate008 gel 002 mtORF after extraction:
20250227 plate008 gel 003 mtORF after extraction:
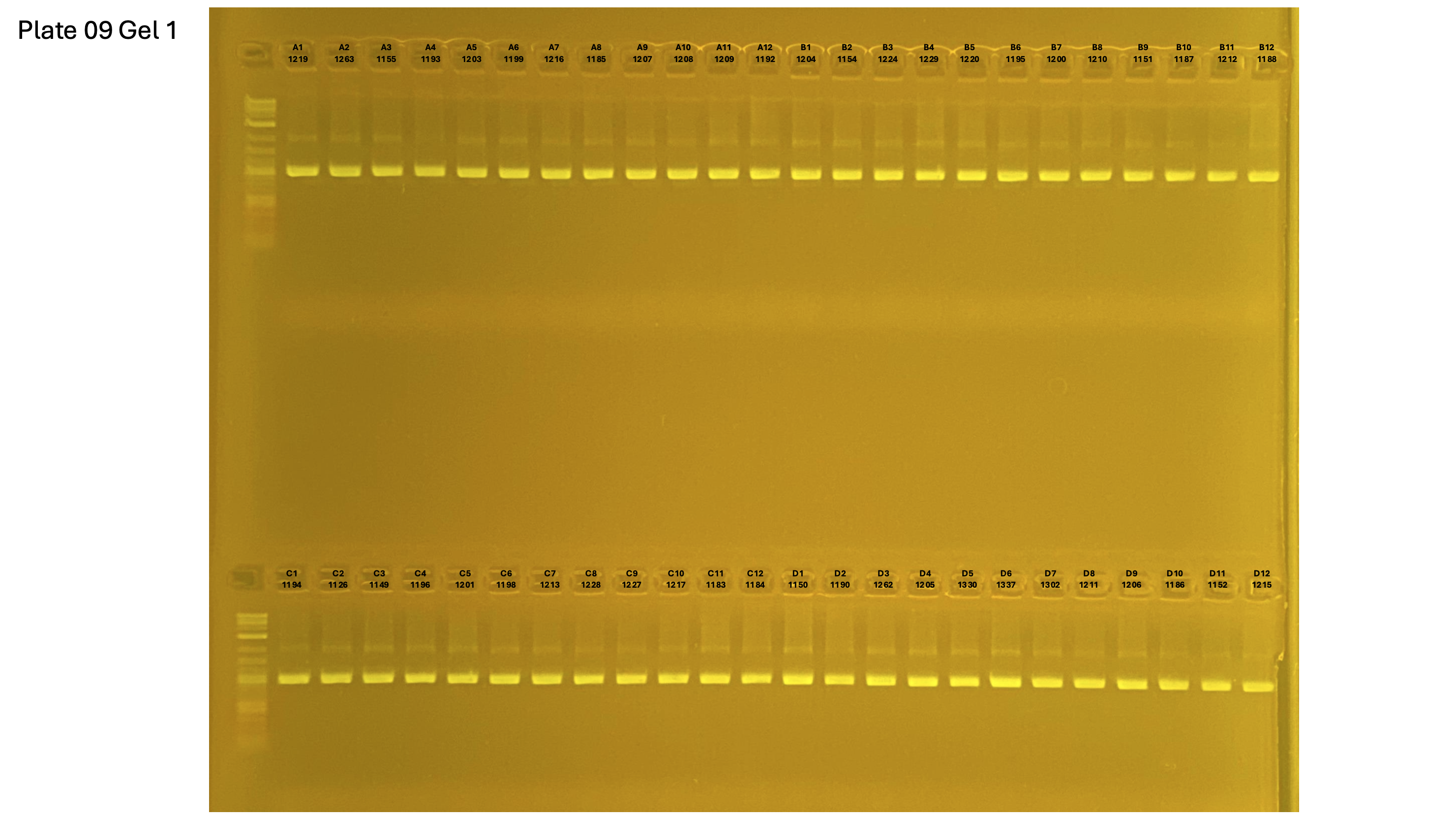
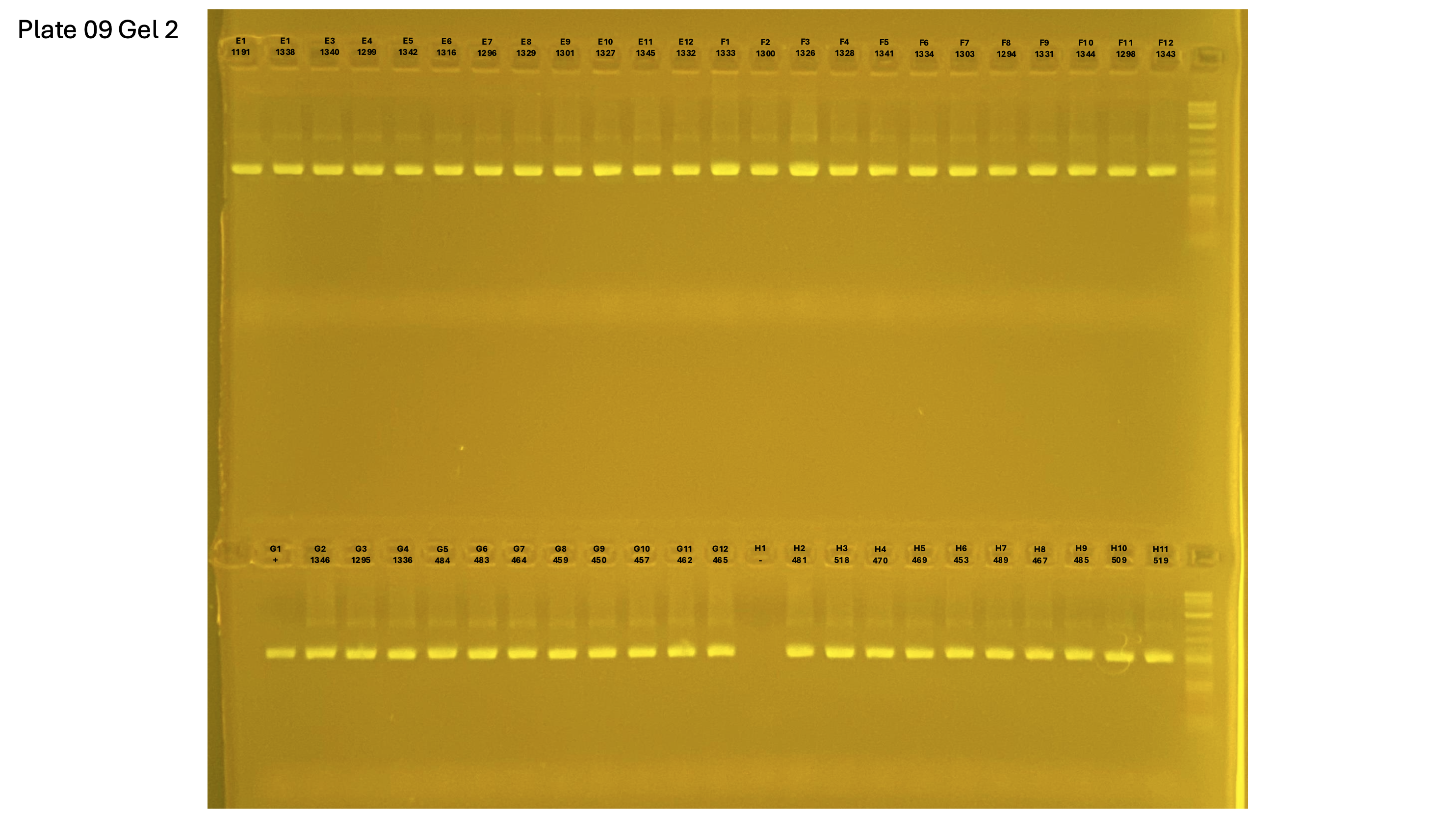
20250227 plate009 gel 001 mtORF after extraction:
20250227 plate009 gel 002 mtORF after extraction:
20250227 plate009 gel 003 mtORF after extraction:
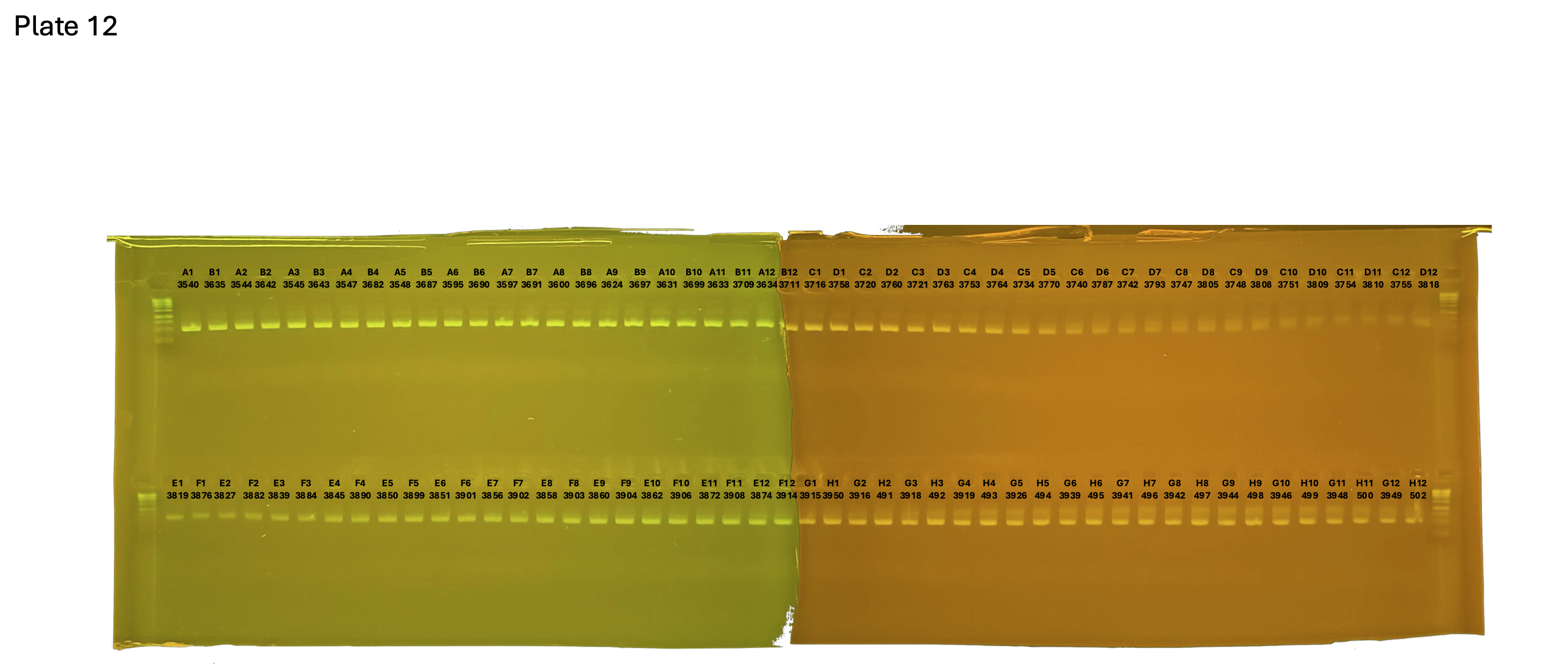
20241005 plate012 mtORF after extraction:
20241005 plate013 mtORF after extraction:
20241005 plate014 mtORF after extraction:
20241005 plate015 mtORF after extraction:
20241005 plate016 mtORF after extraction:
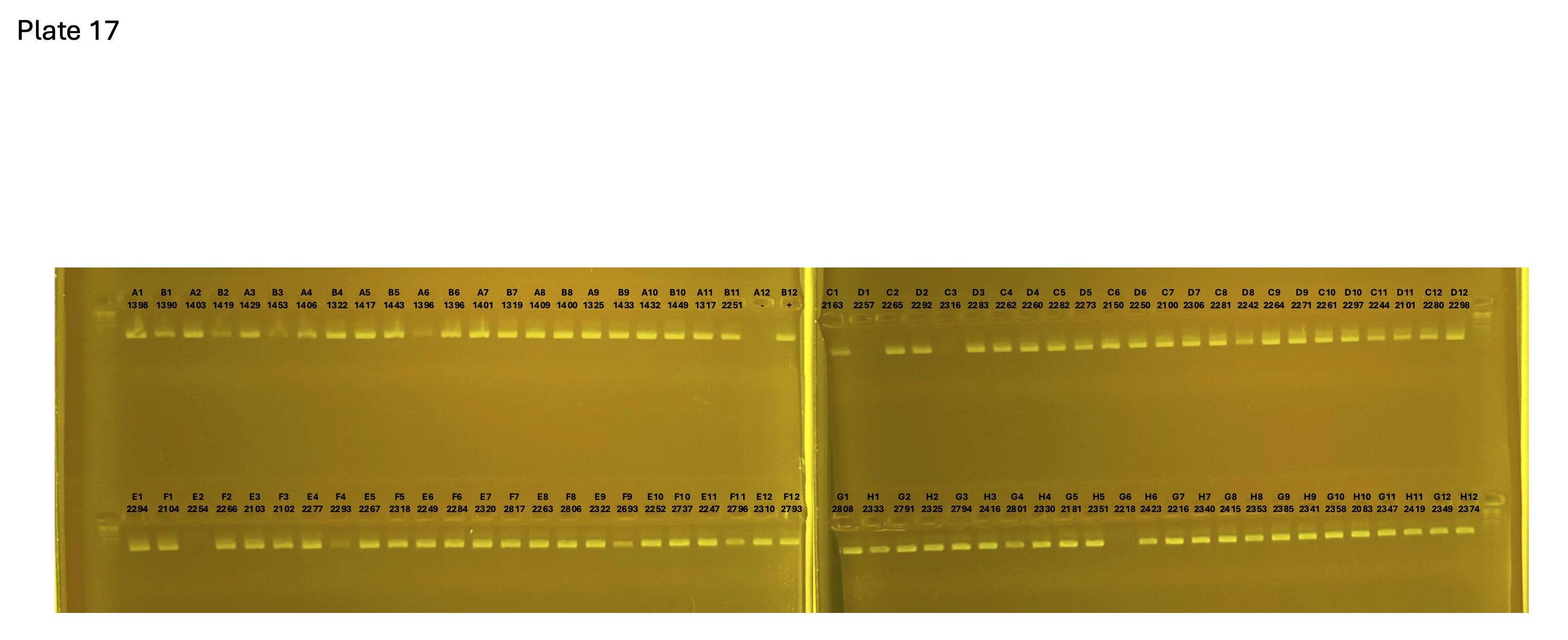
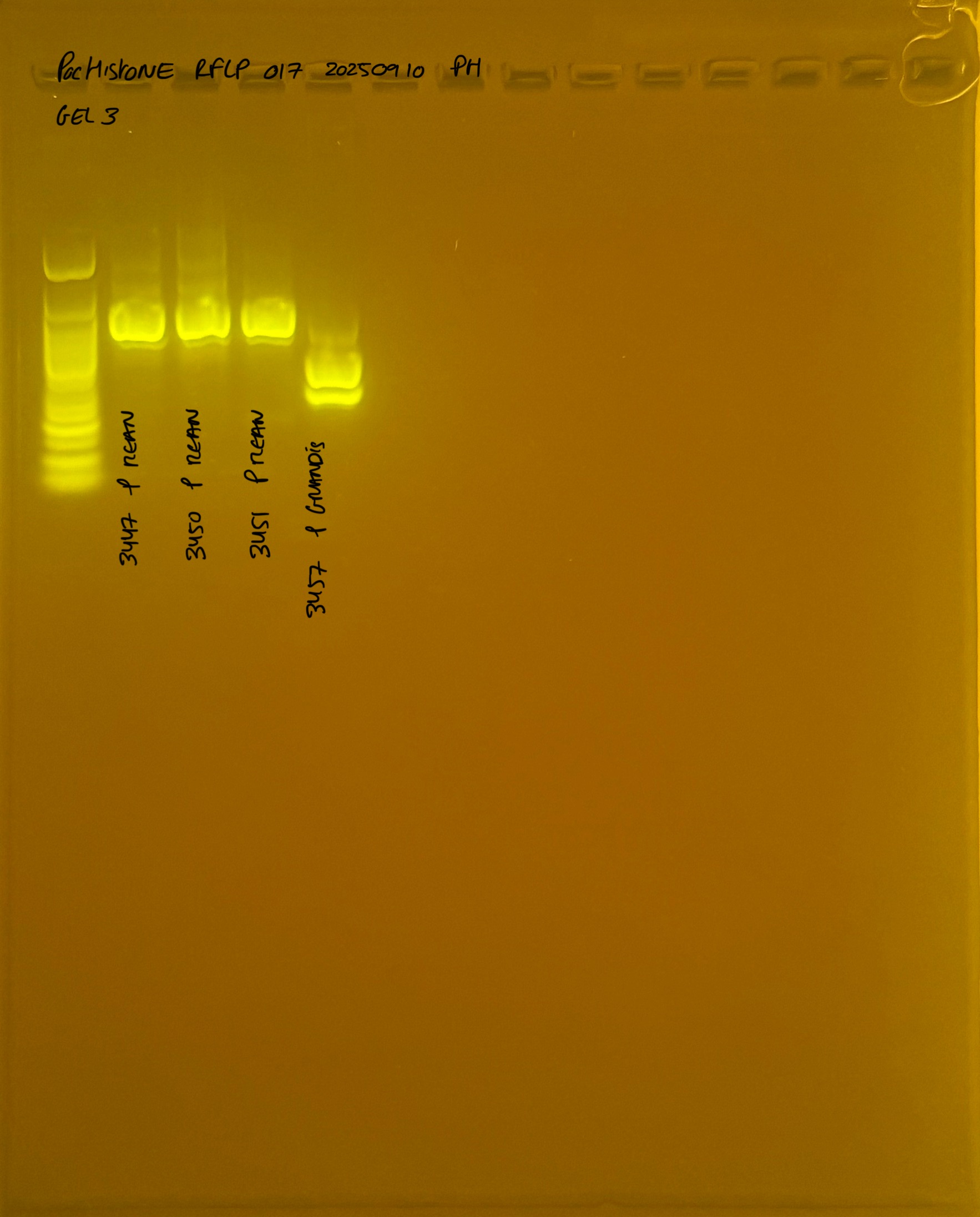
2024xxxx plate017 mtORF after extraction:
20241005 plate018 mtORF after extraction:
2024xxxx plate019 mtORF after extraction:
2024xxxx plate020 gel 001 mtORF after extraction:
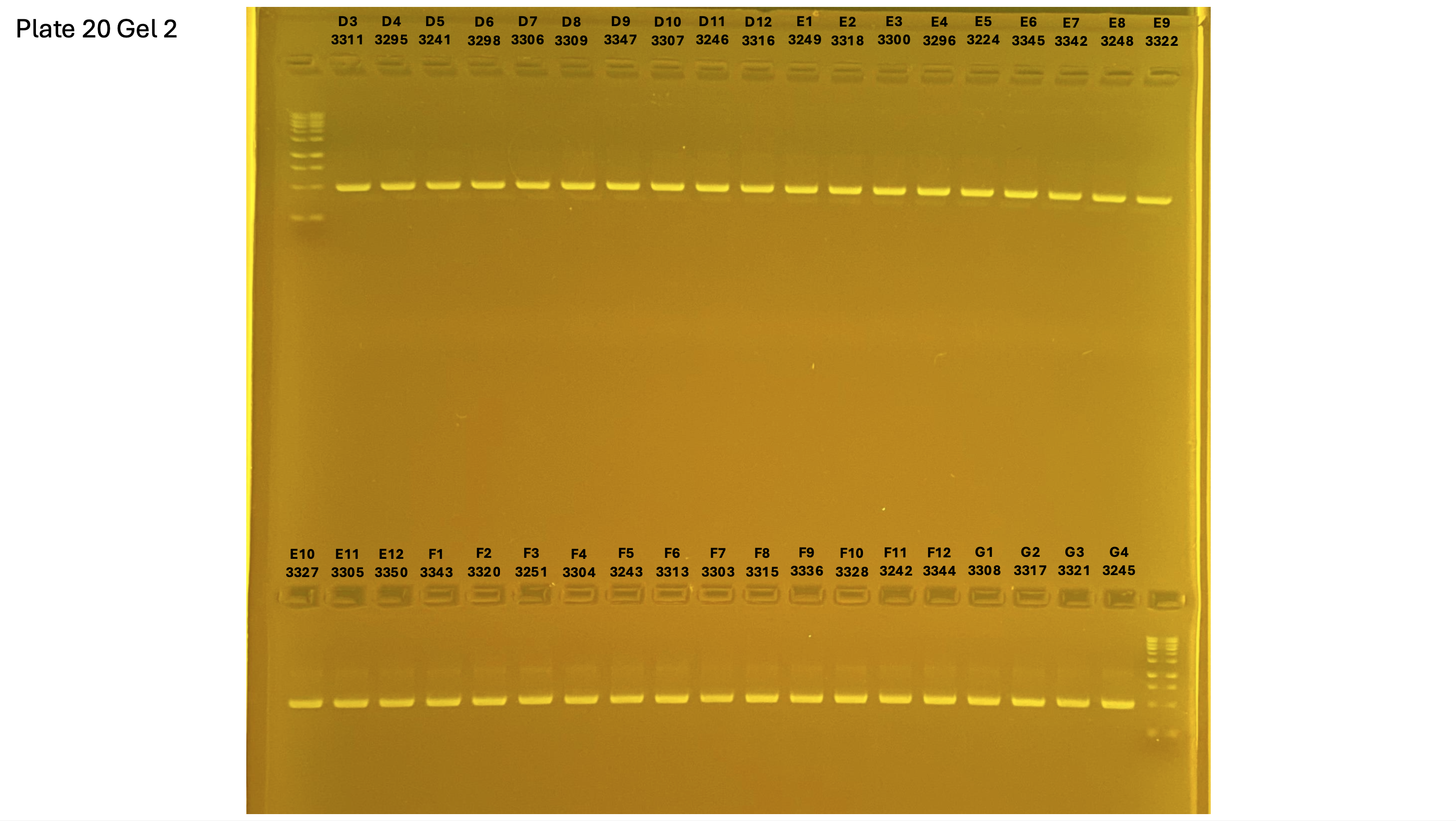
2024xxxx plate020 gel 002 mtORF after extraction:
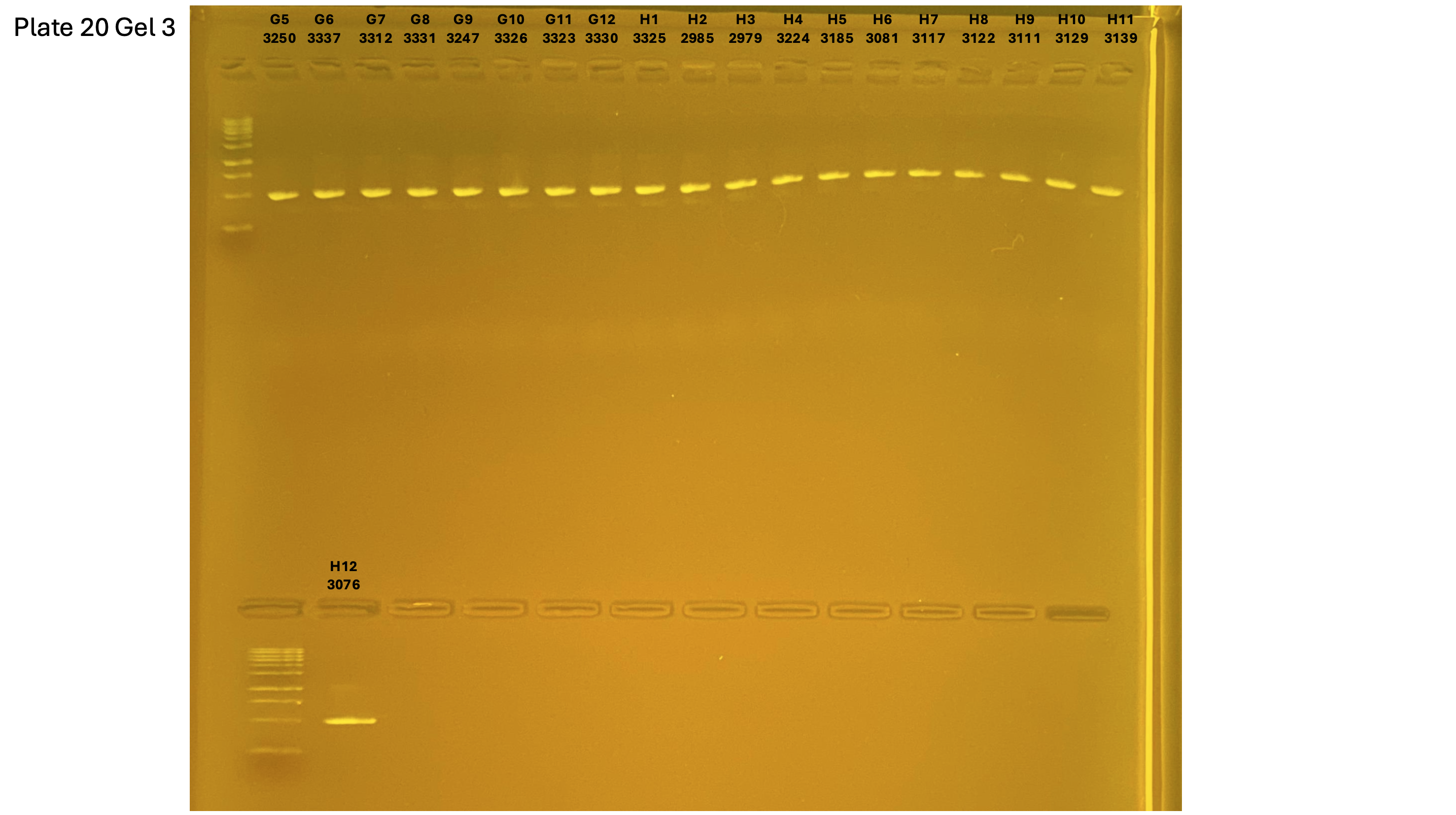
2024xxxx plate020 gel 003 mtORF after extraction:
2024xxxx plate021 gel 001 mtORF after extraction:
2024xxxx plate021 gel 002 mtORF after extraction:
2024xxxx plate021 gel 003 mtORF after extraction:
20240928 plate022 gel 001 mtORF after extraction:
20240928 plate022 gel 002 mtORF after extraction:
20240928 plate022 gel 003 mtORF after extraction:
20240928 plate023 gel 001 mtORF after extraction:
20240928 plate023 gel 002 mtORF after extraction:
20240928 plate023 gel 003 mtORF after extraction:
20240930 plate024 gel 001 mtORF after extraction:
20240930 plate024 gel 002 mtORF after extraction:
20240930 plate024 gel 003 mtORF after extraction:
20240930 plate025 gel 001 mtORF after extraction:
20240930 plate025 gel 002 mtORF after extraction:
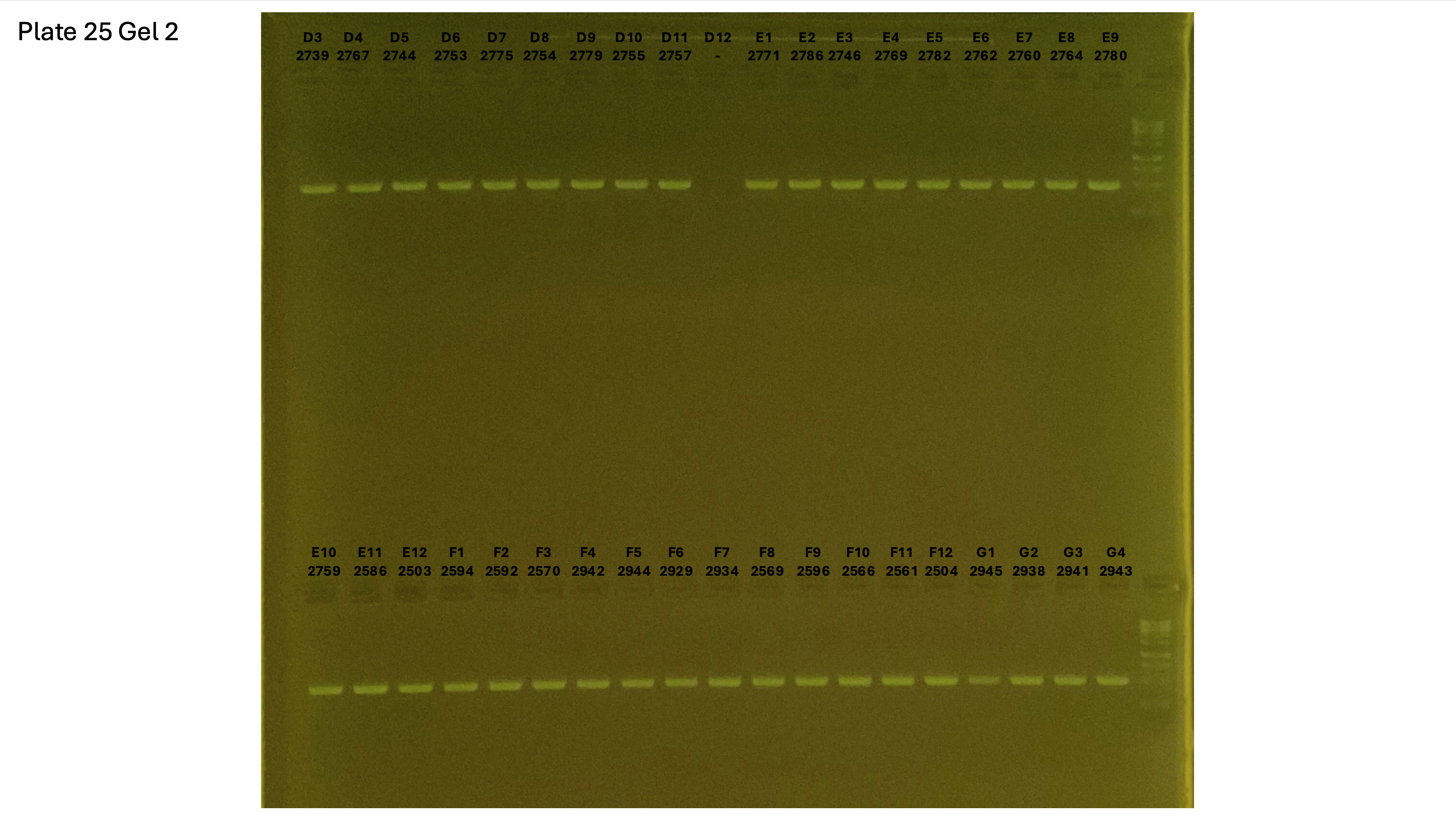
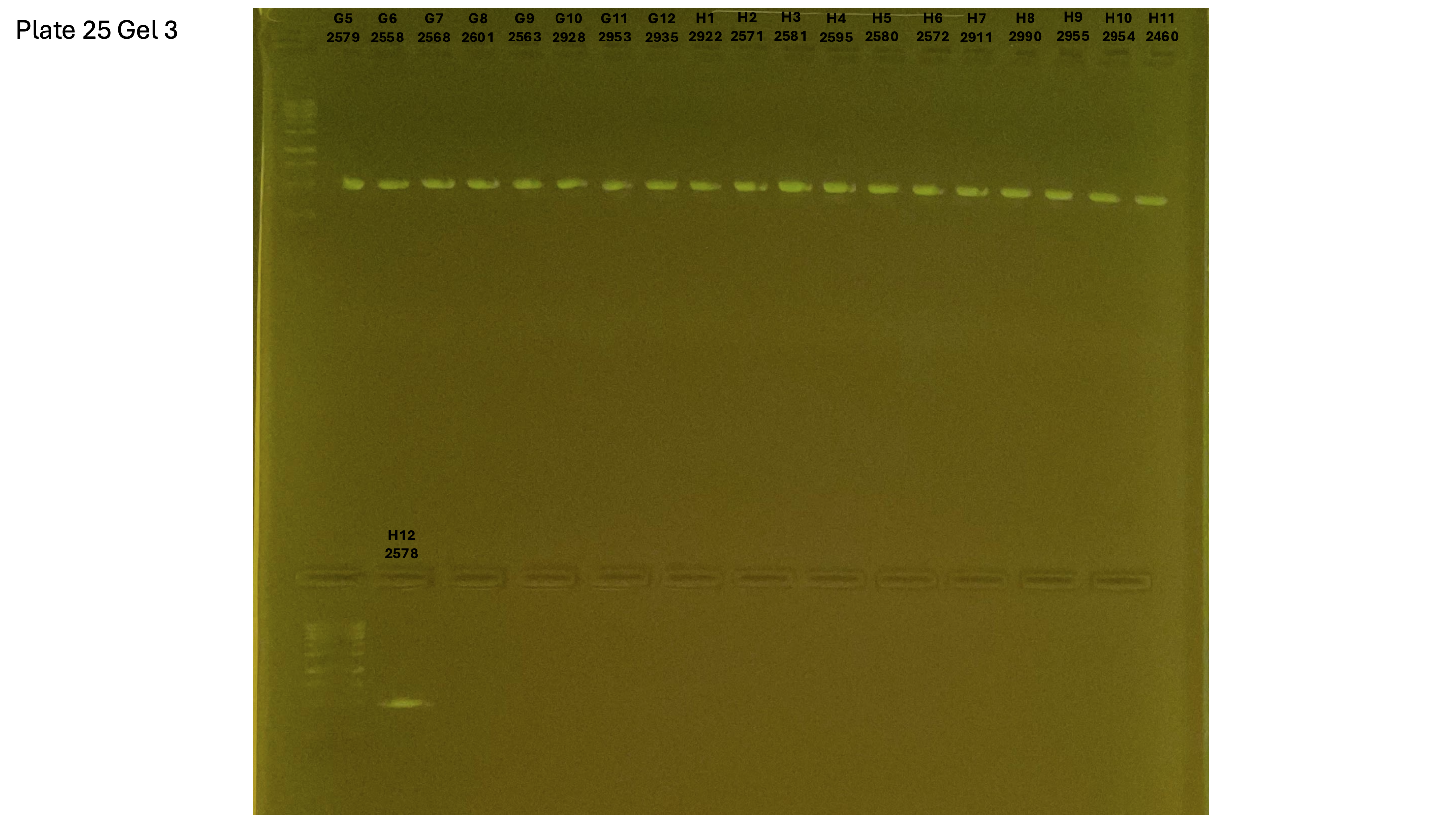
20240930 plate025 gel 003 mtORF after extraction:
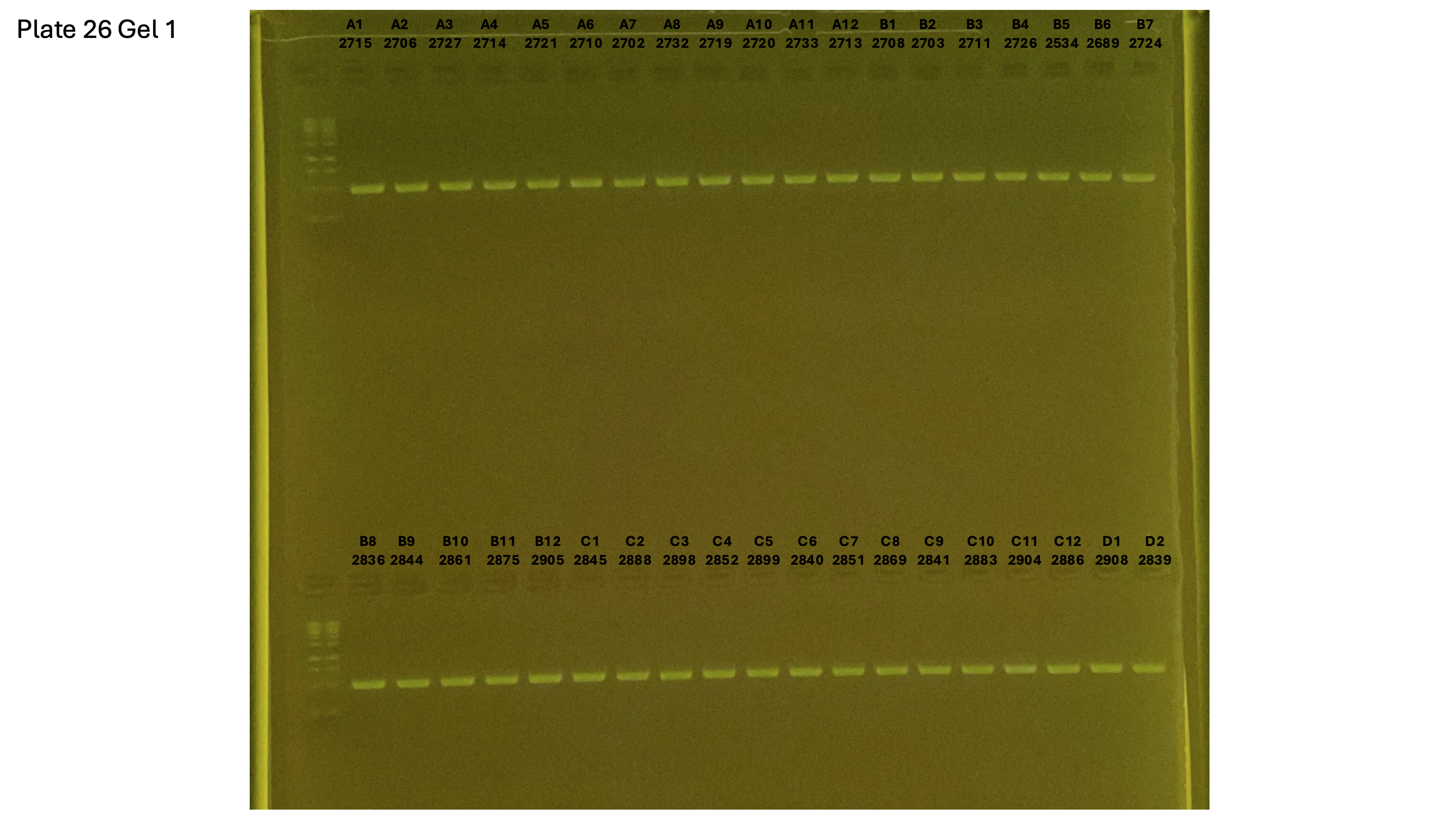
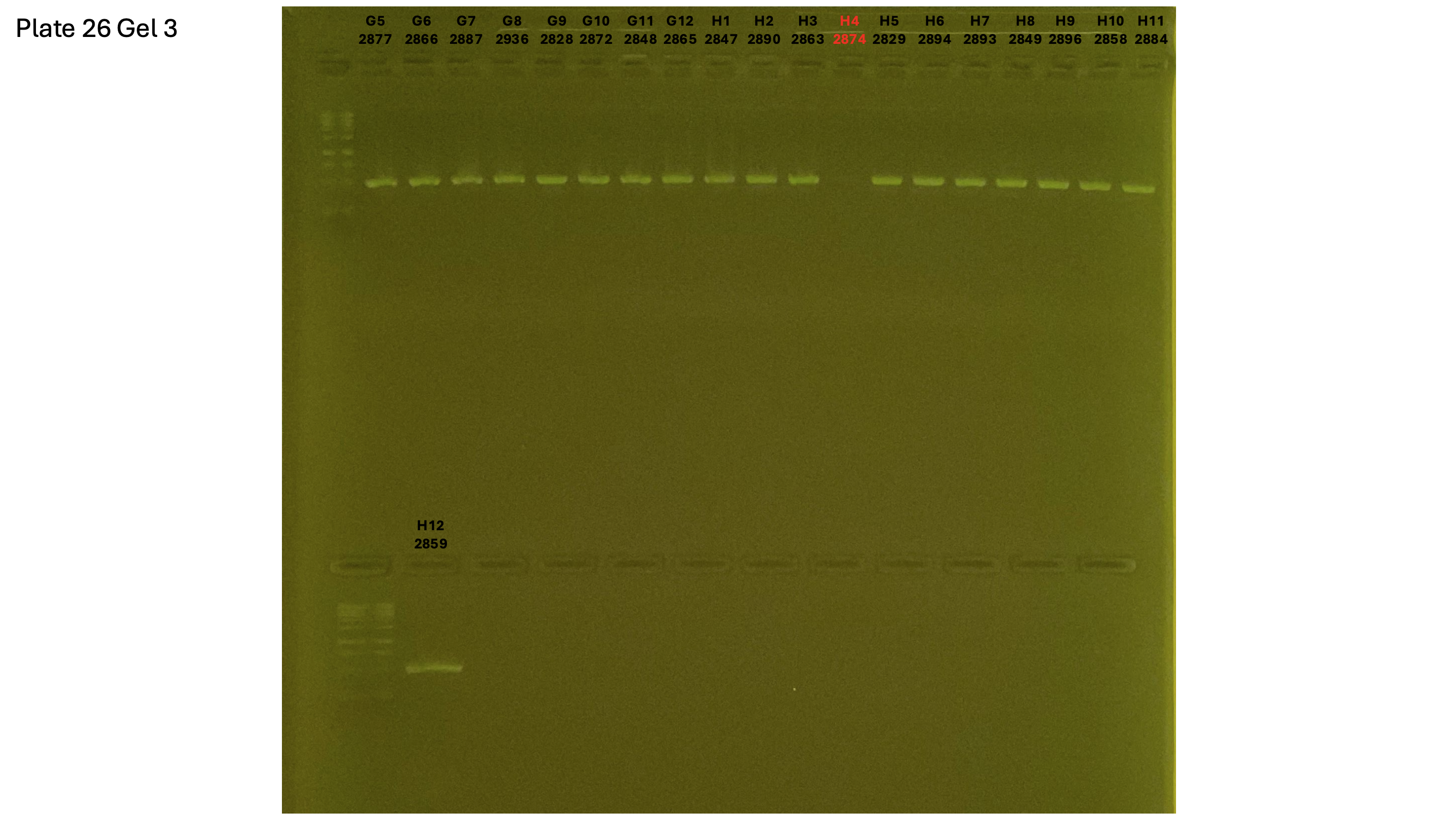
20240930 plate026 gel 001 mtORF after extraction:
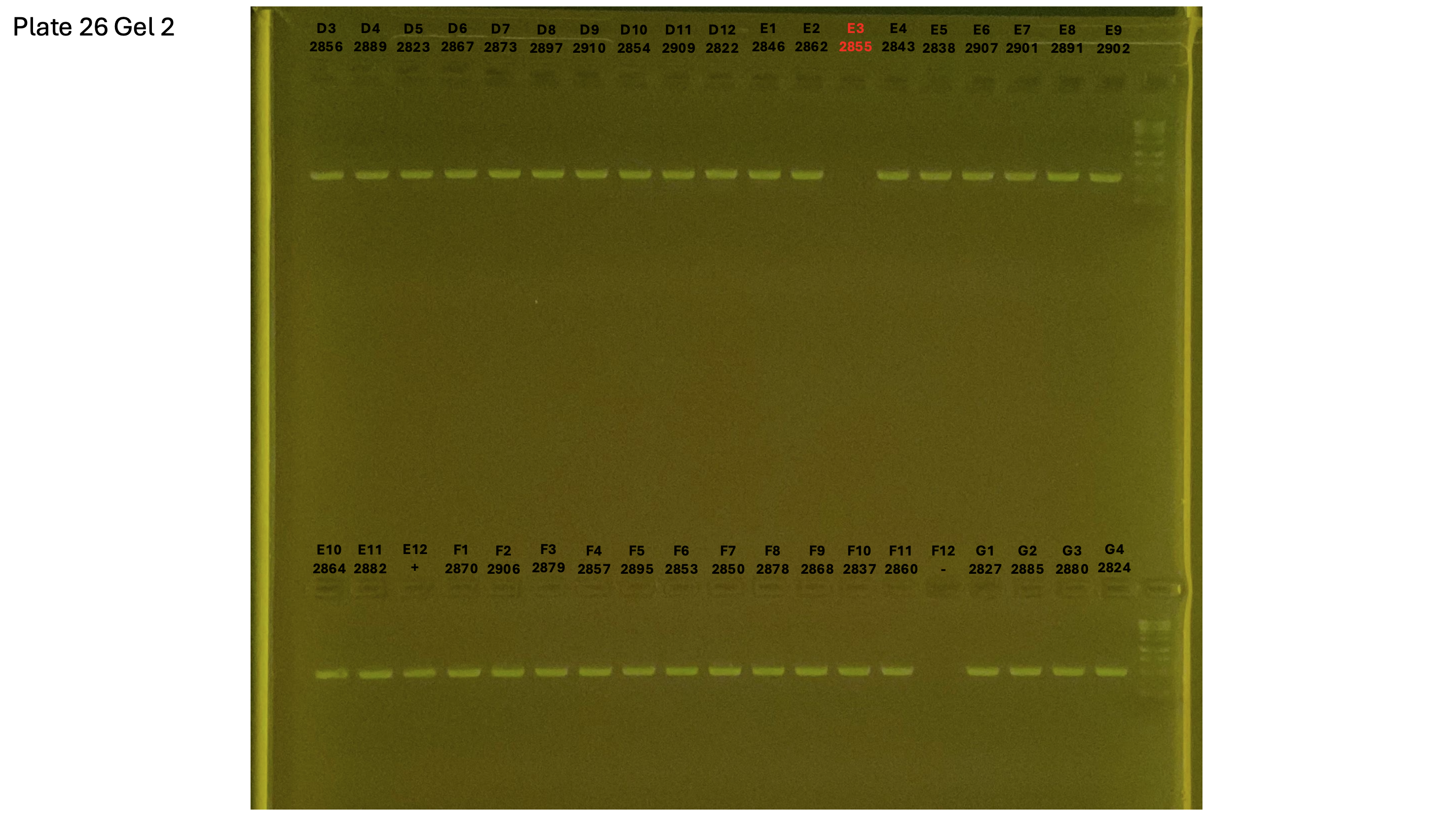
20240930 plate026 gel 002 mtORF after extraction:
20240930 plate026 gel 003 mtORF after extraction:
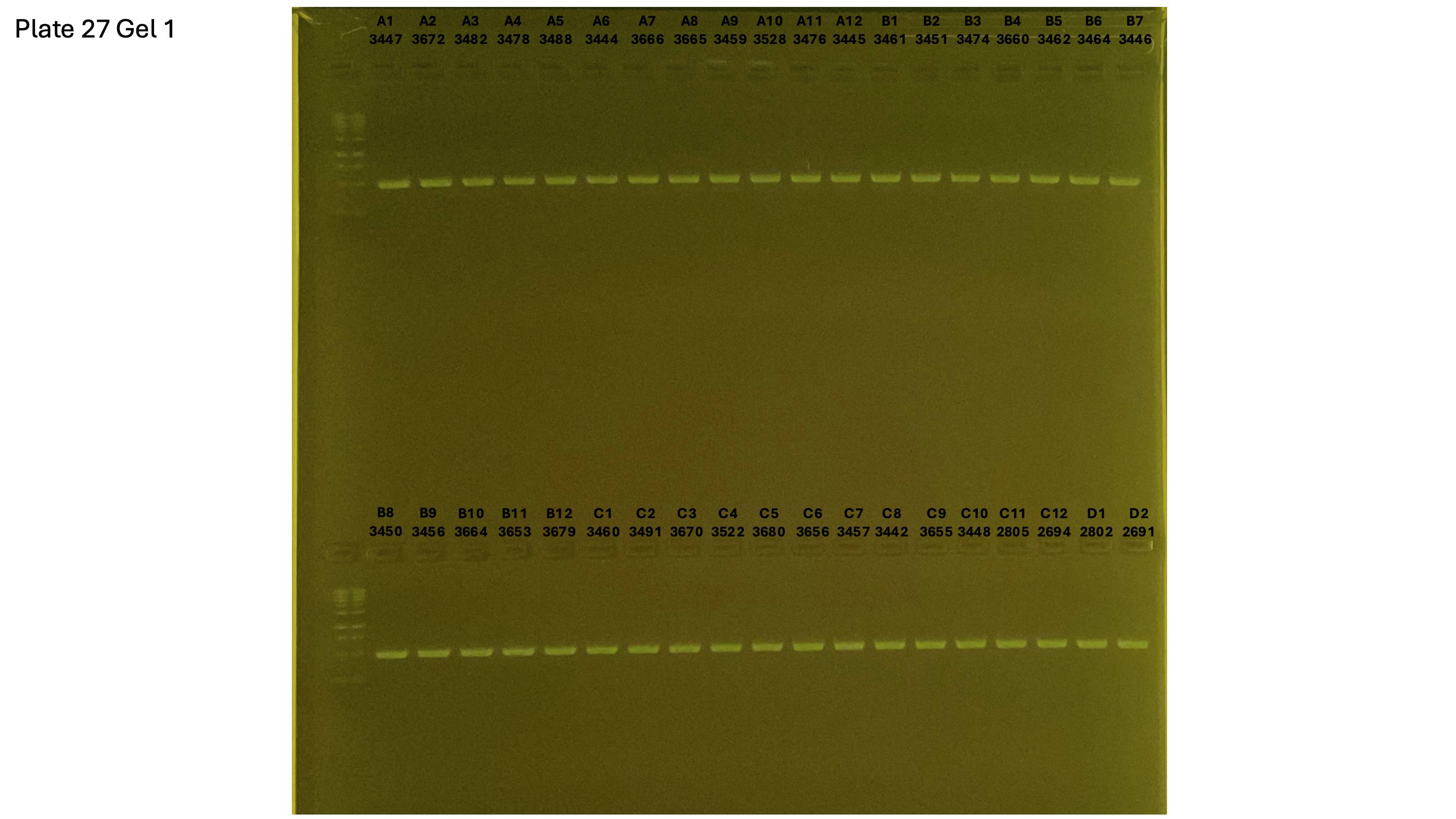
20240930 plate027 gel 001 mtORF after extraction:
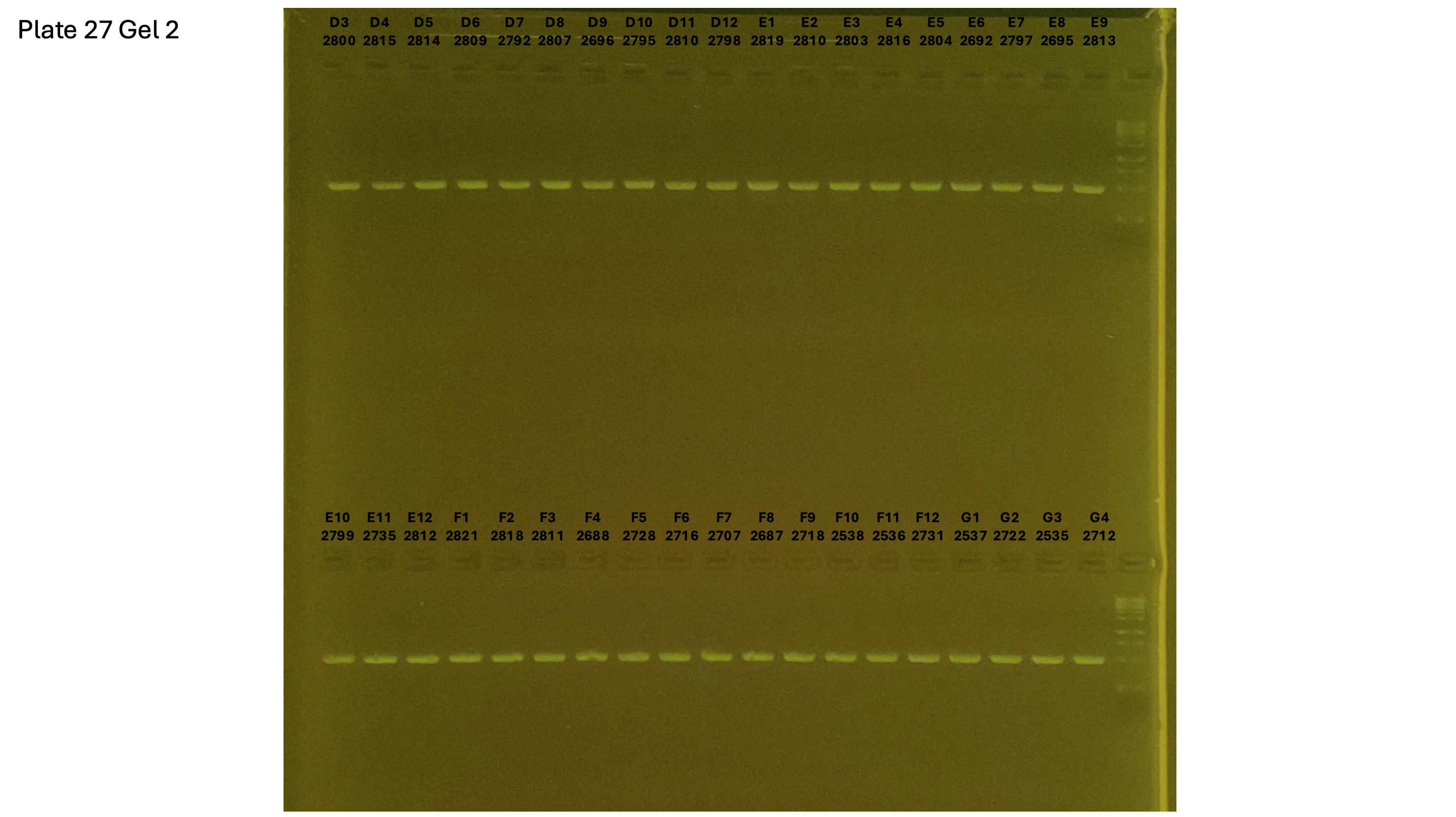
20240930 plate027 gel 002 mtORF after extraction:
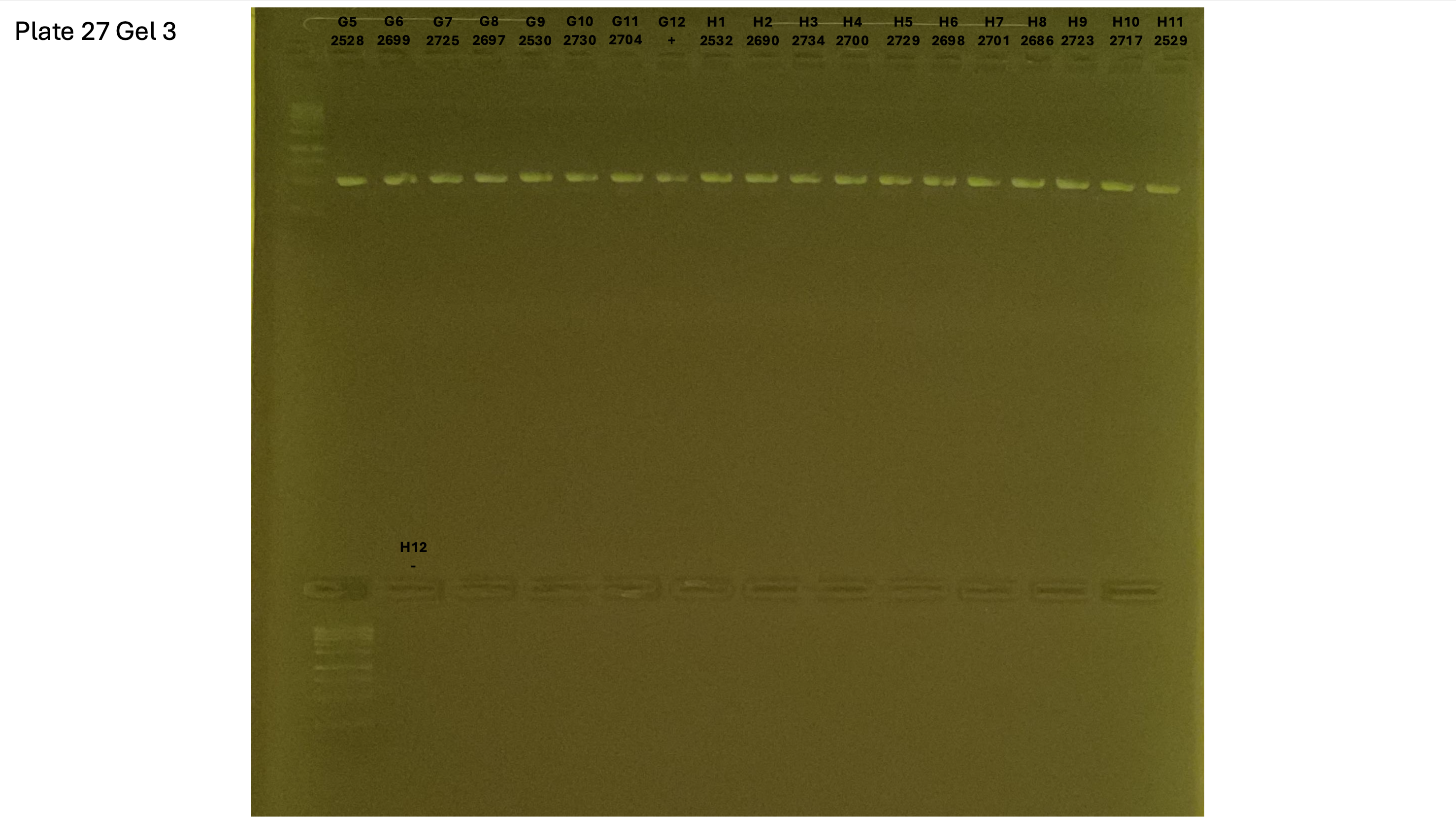
20240930 plate027 gel 003 mtORF after extraction:
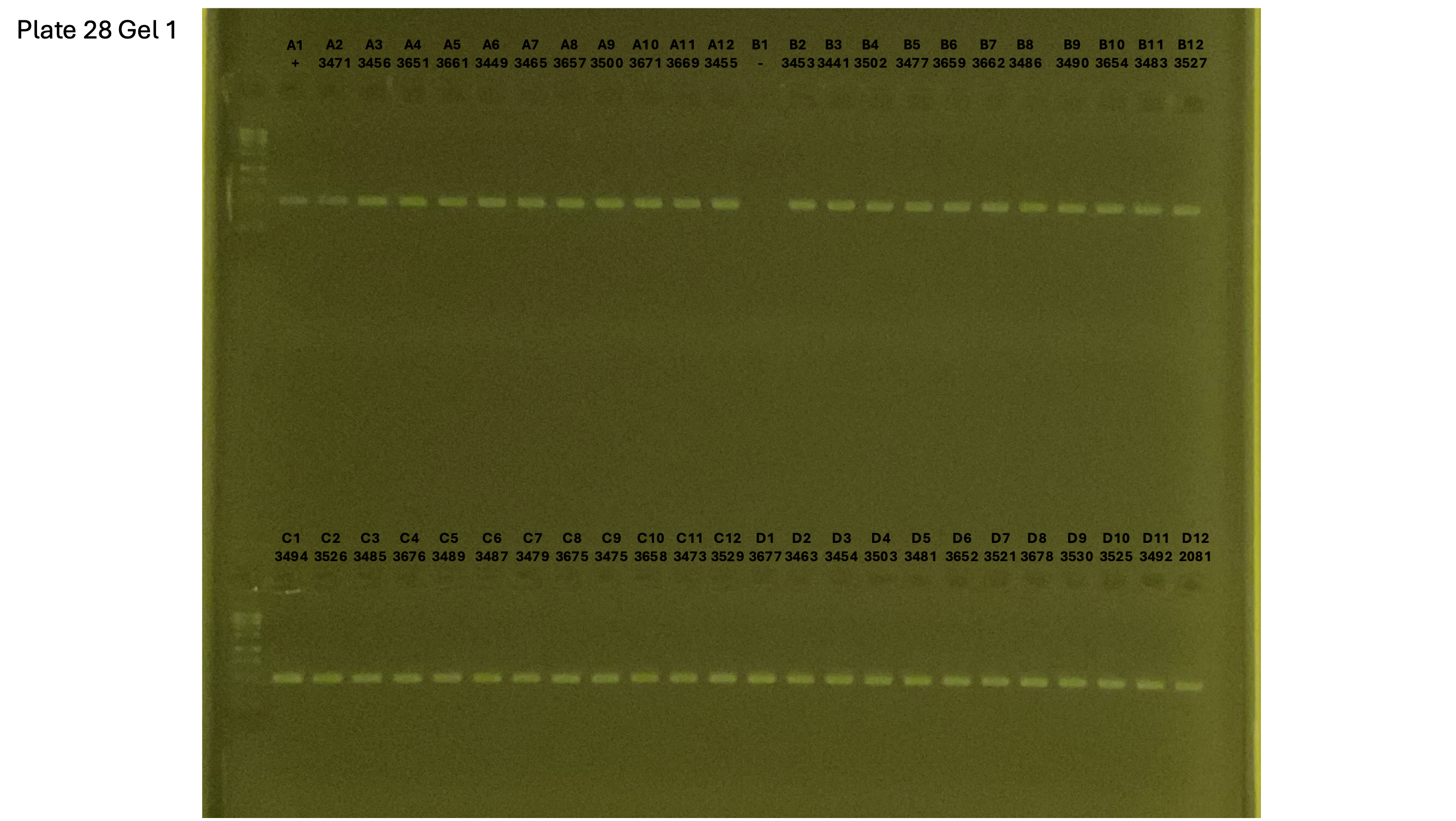
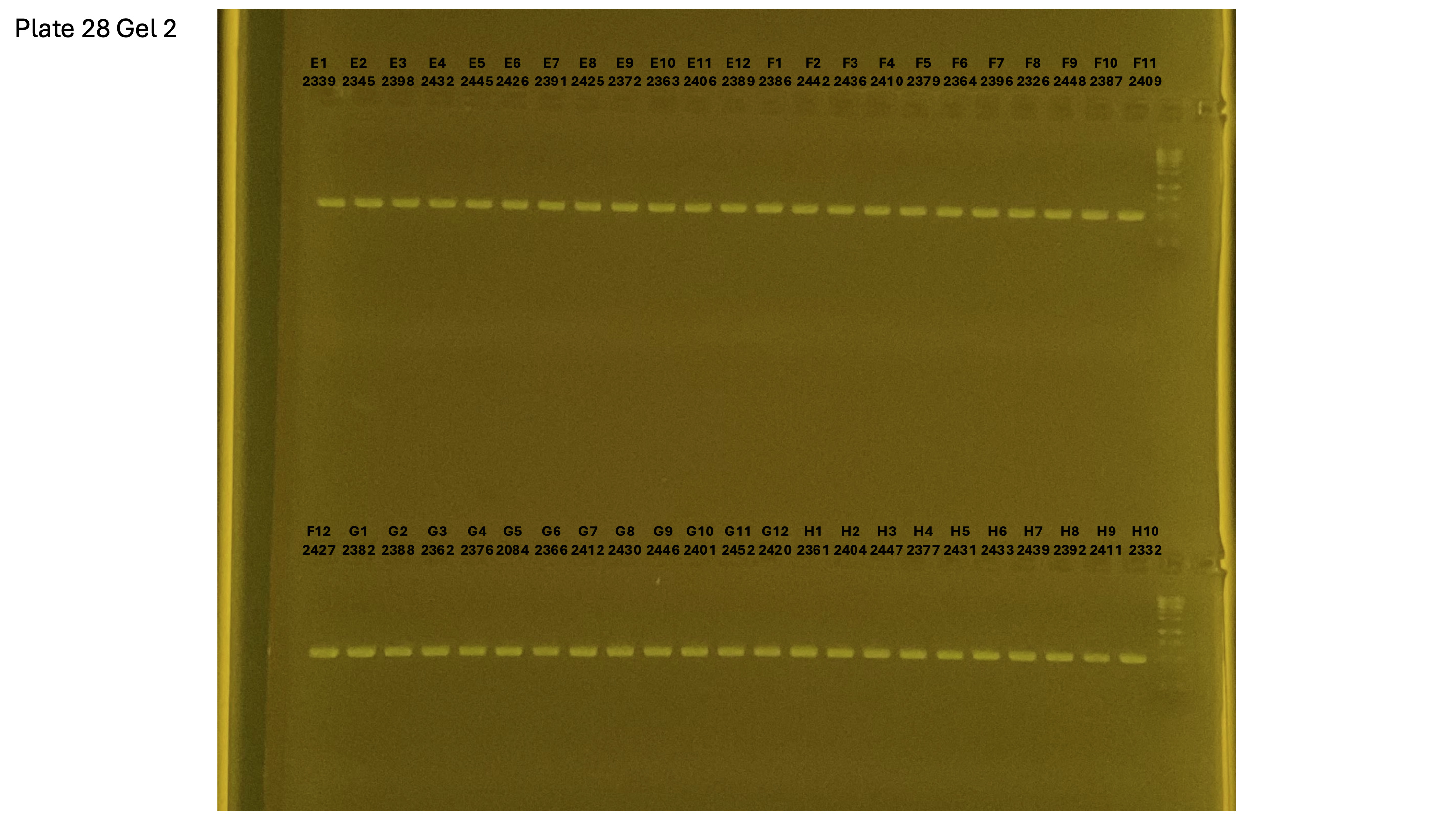
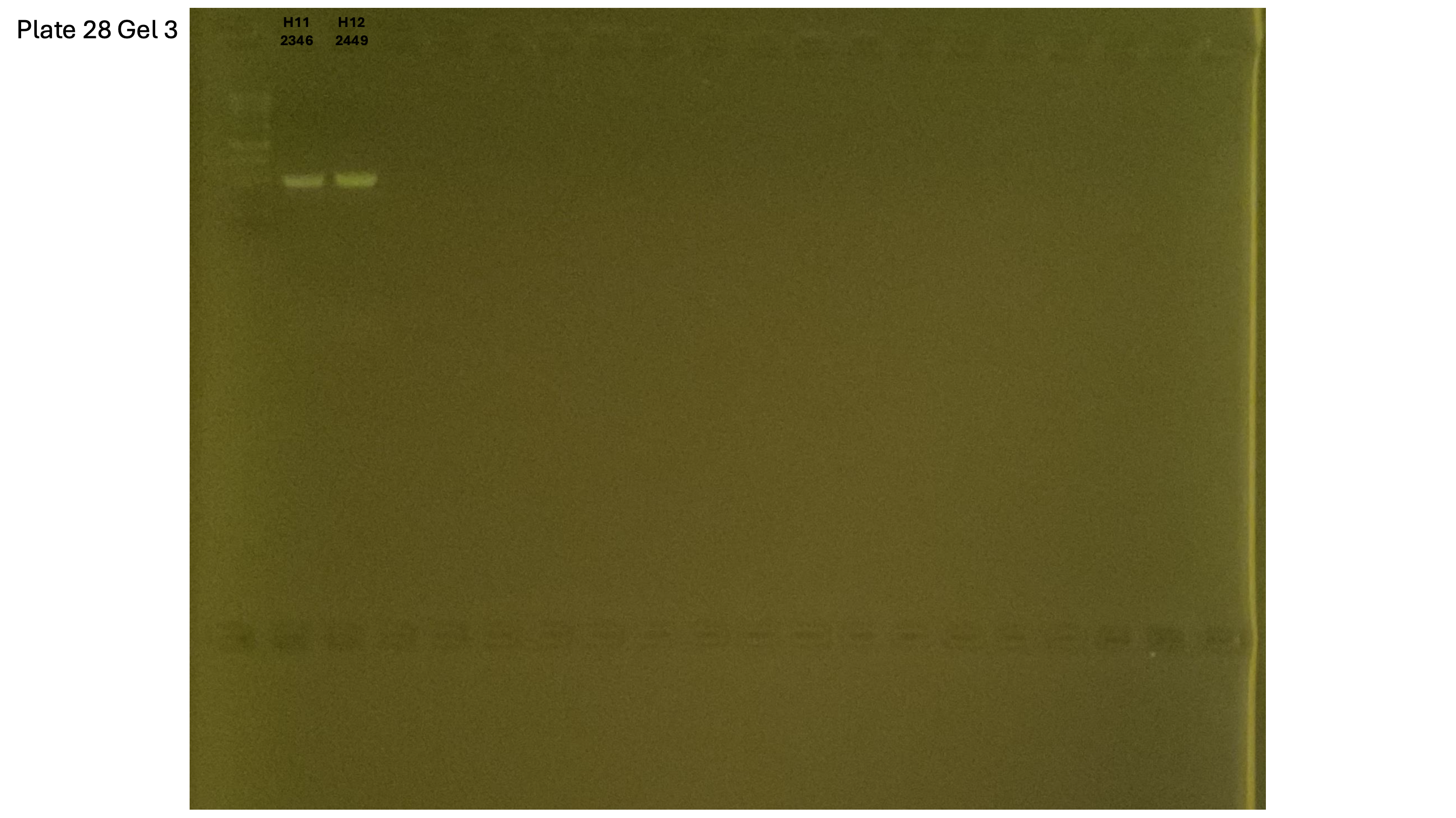
20241001 plate028 gel 001 mtORF after extraction:
20241001 plate028 gel 002 mtORF after extraction:
20241001 plate028 gel 003 mtORF after extraction:
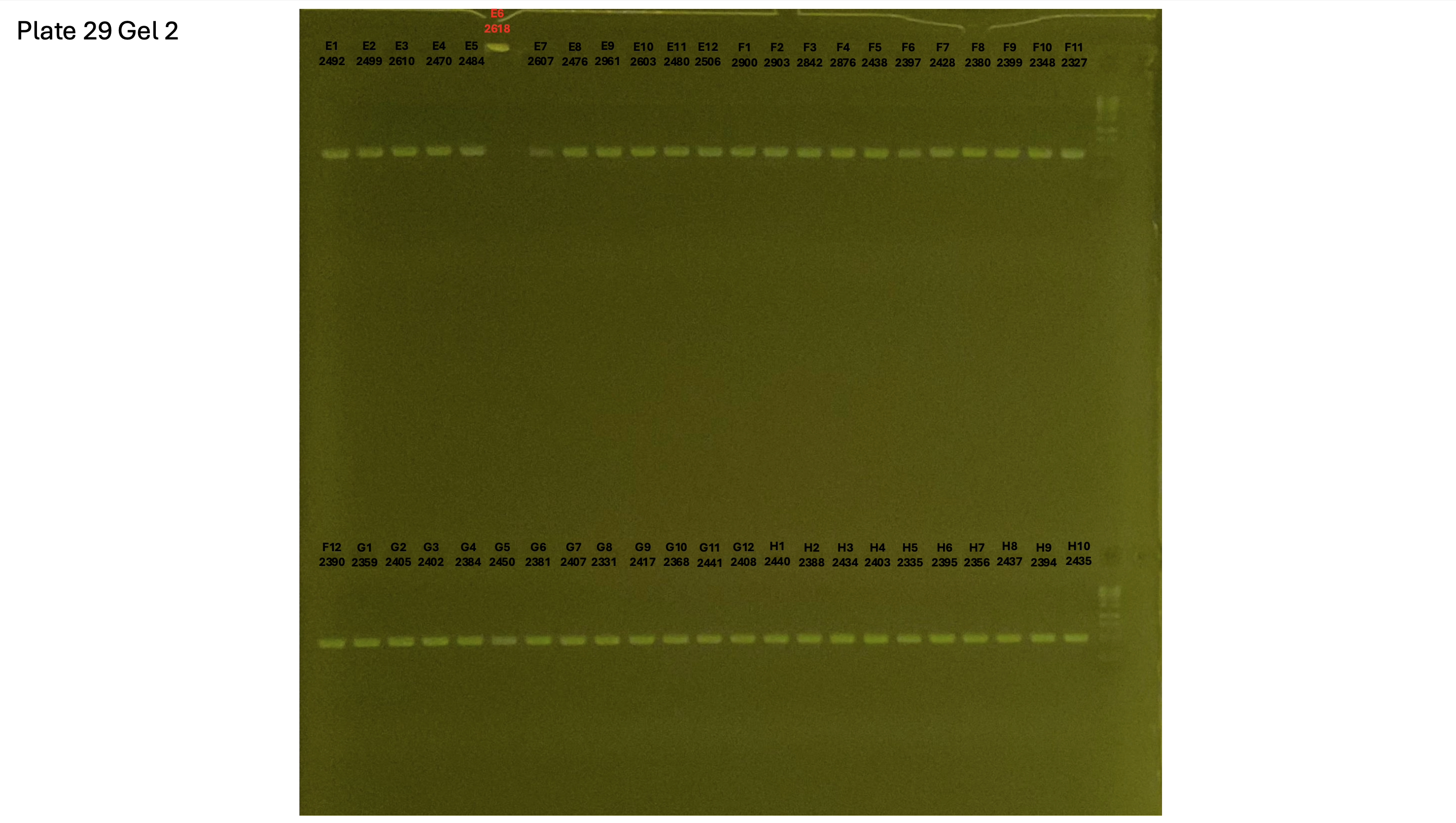
20241001 plate029 gel 001 mtORF after extraction:
20241001 plate029 gel 002 mtORF after extraction:
20241001 plate029 gel 003 mtORF after extraction:
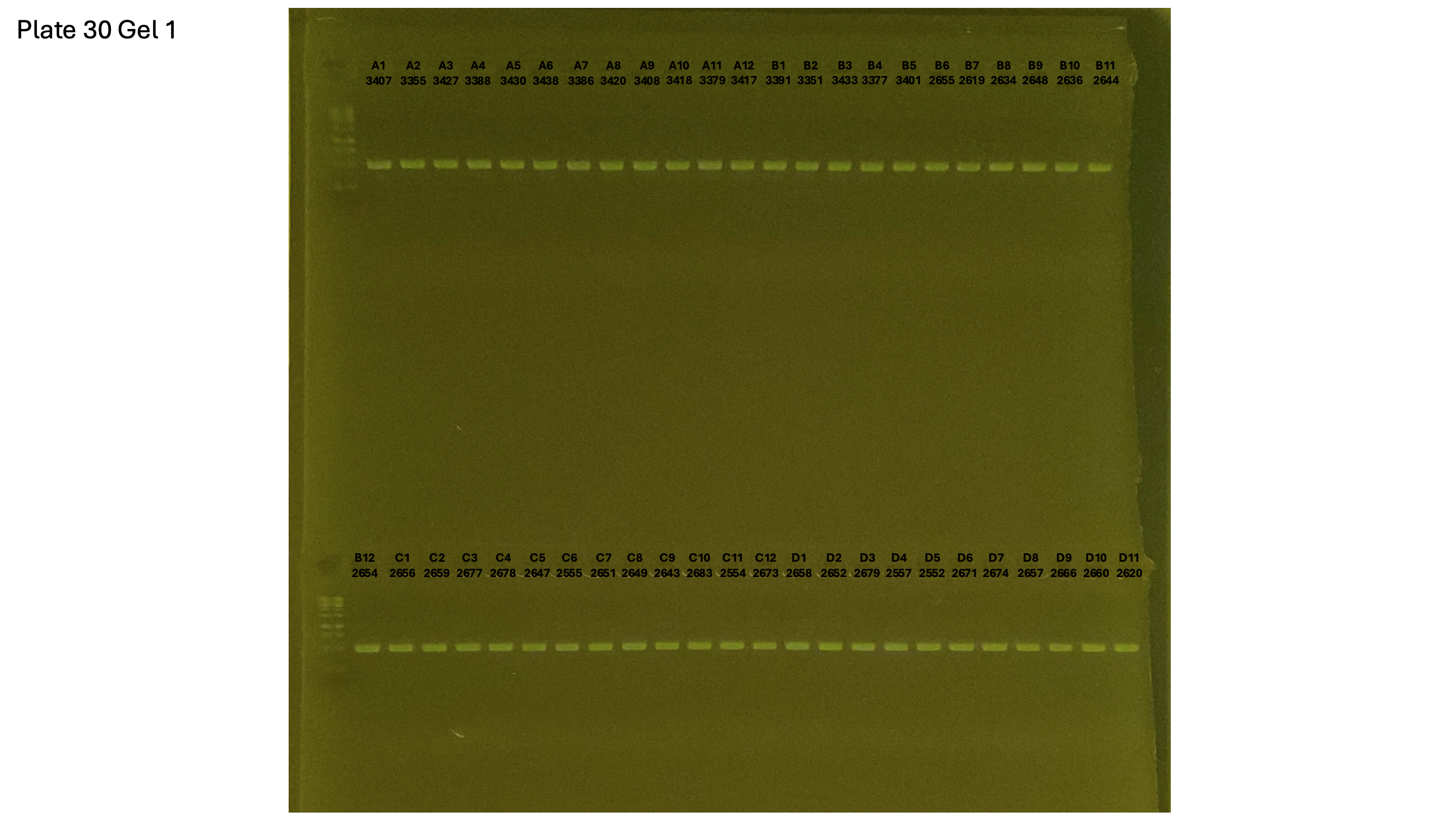
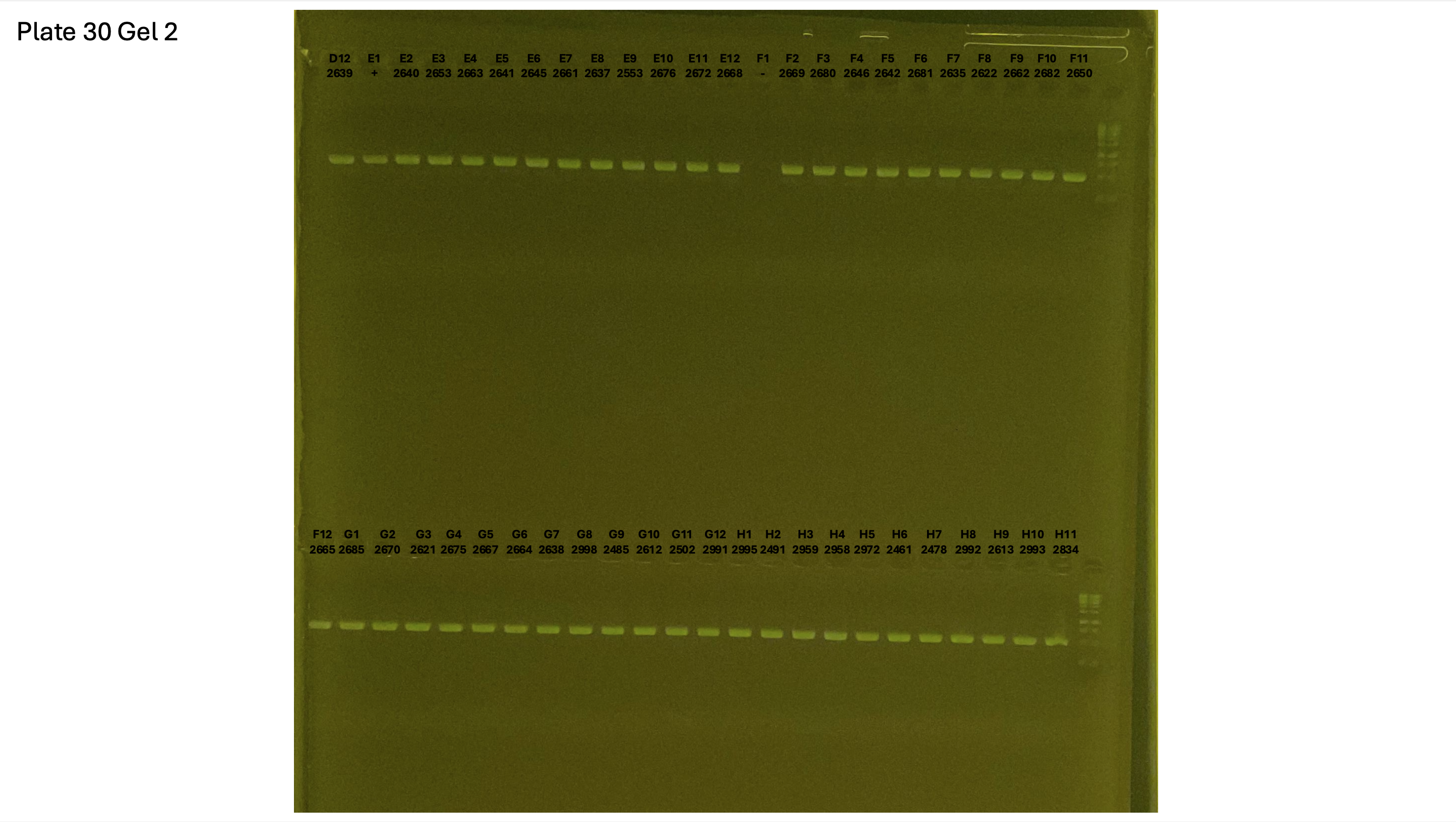
20241001 plate030 gel 001 mtORF after extraction:
20241001 plate030 gel 002 mtORF after extraction:
20241001 plate030 gel 003 mtORF after extraction:
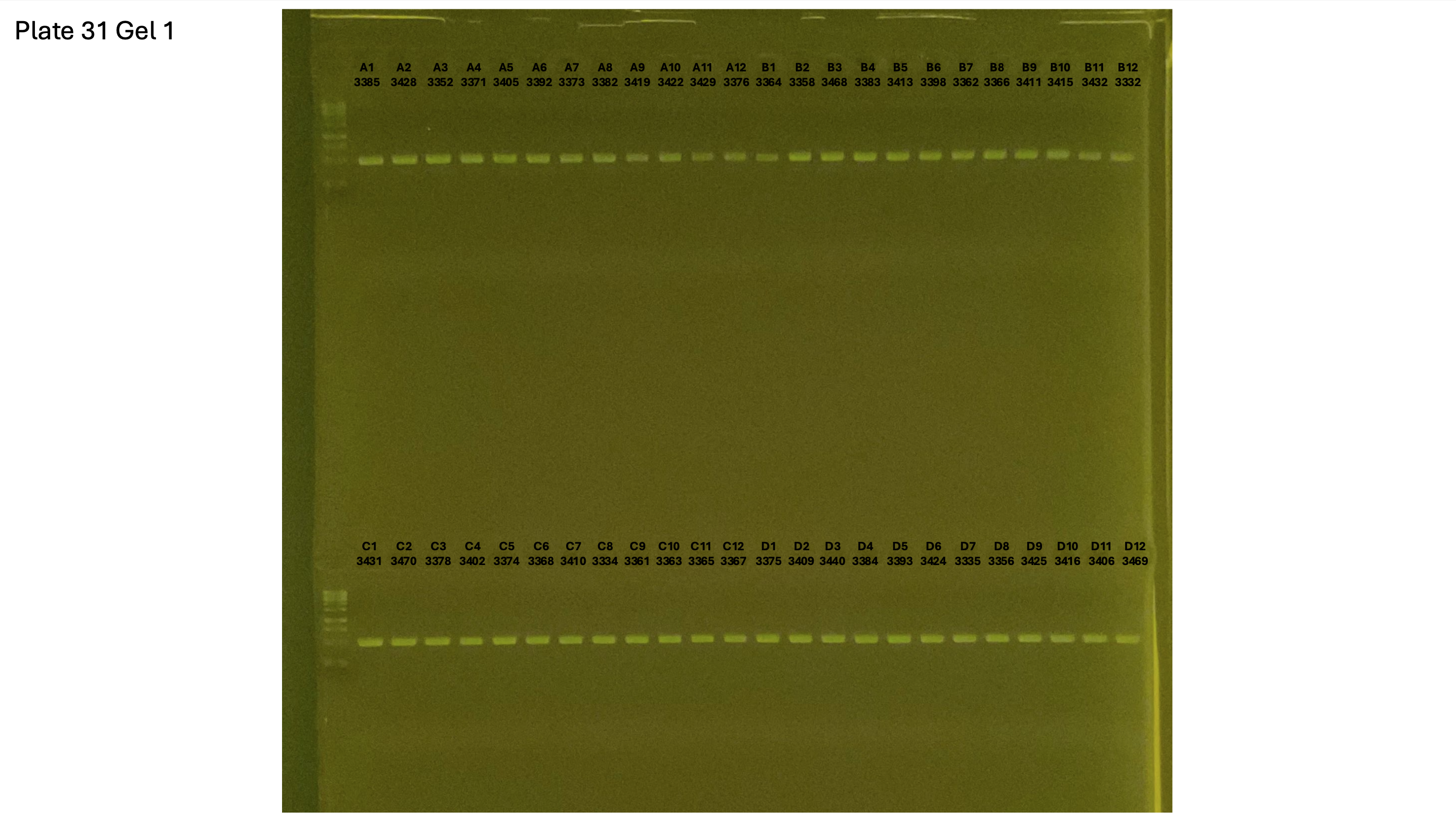
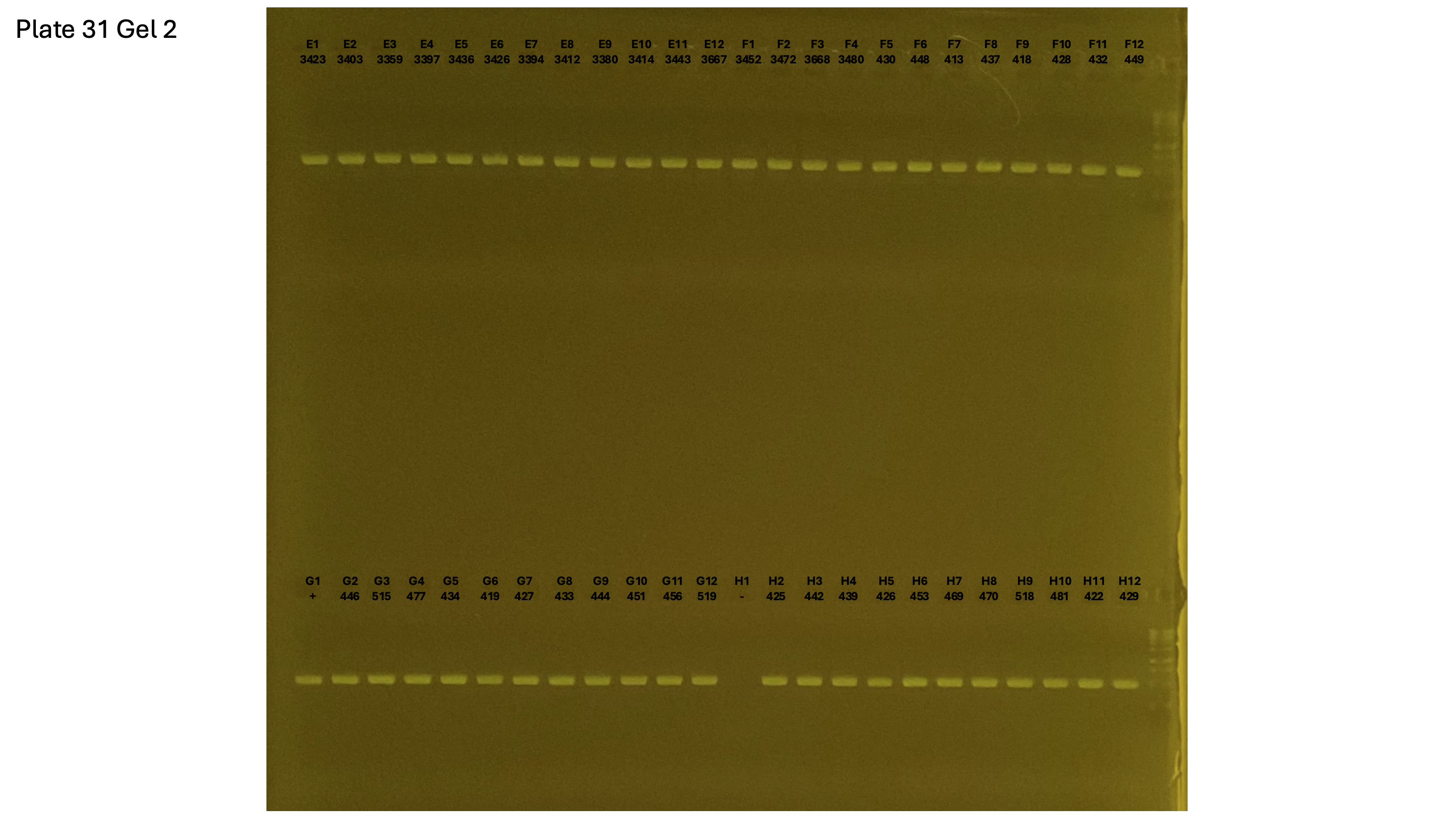
20241001 plate031 gel 001 mtORF after extraction:
20241001 plate031 gel 002 mtORF after extraction:
20241001 plate032 gel 001 mtORF after extraction:
20241001 plate032 gel 002 mtORF after extraction:
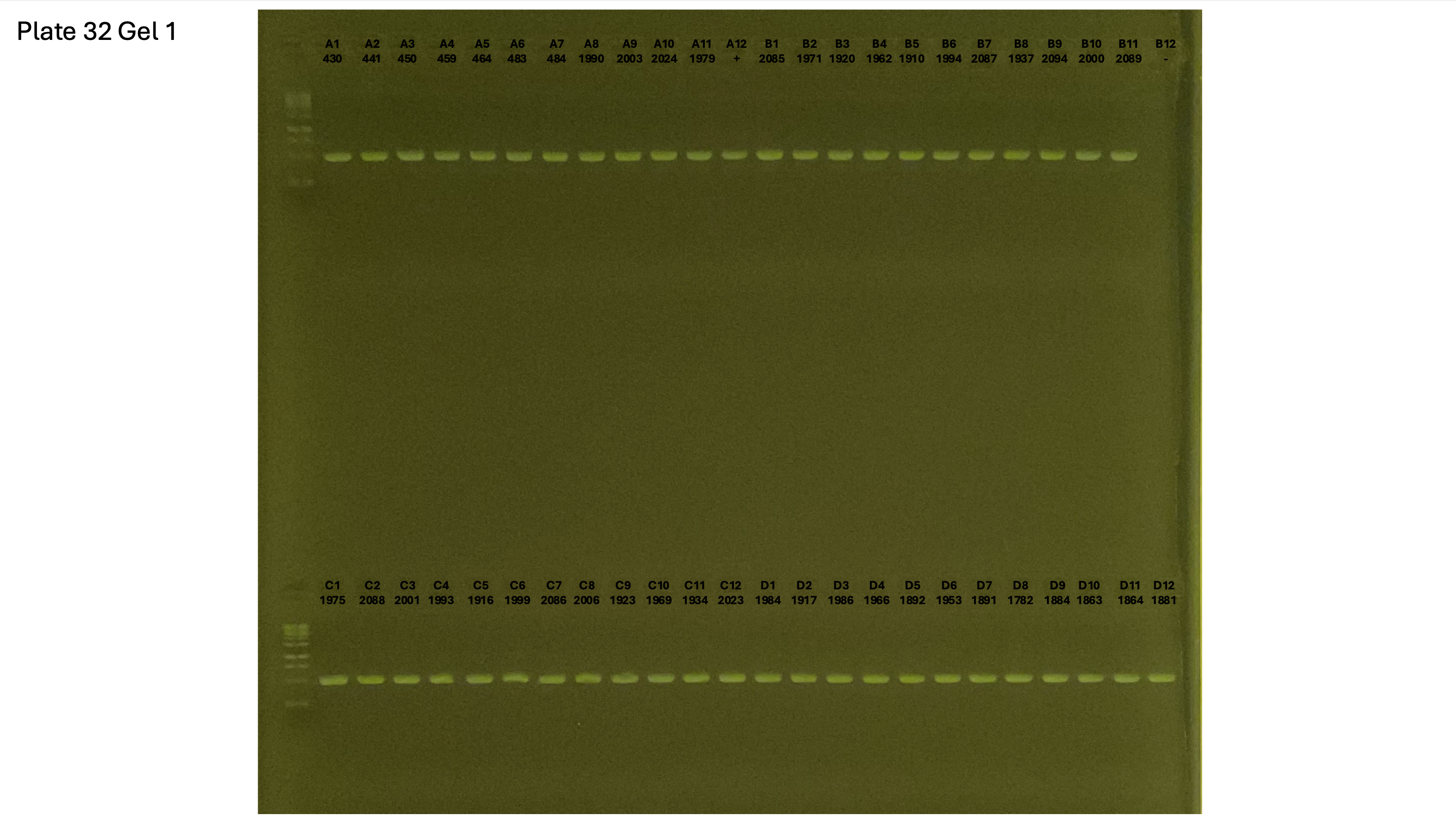
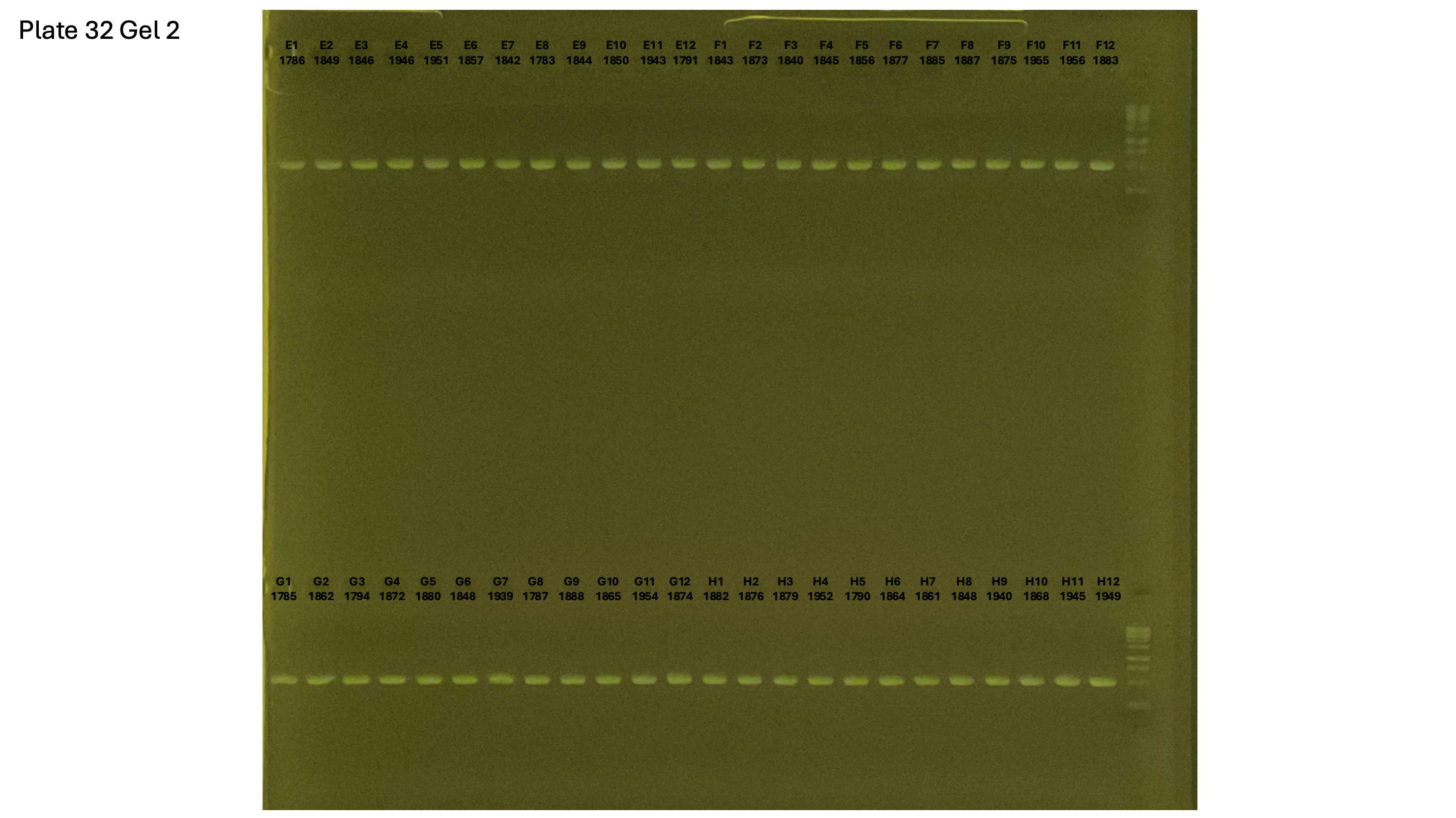
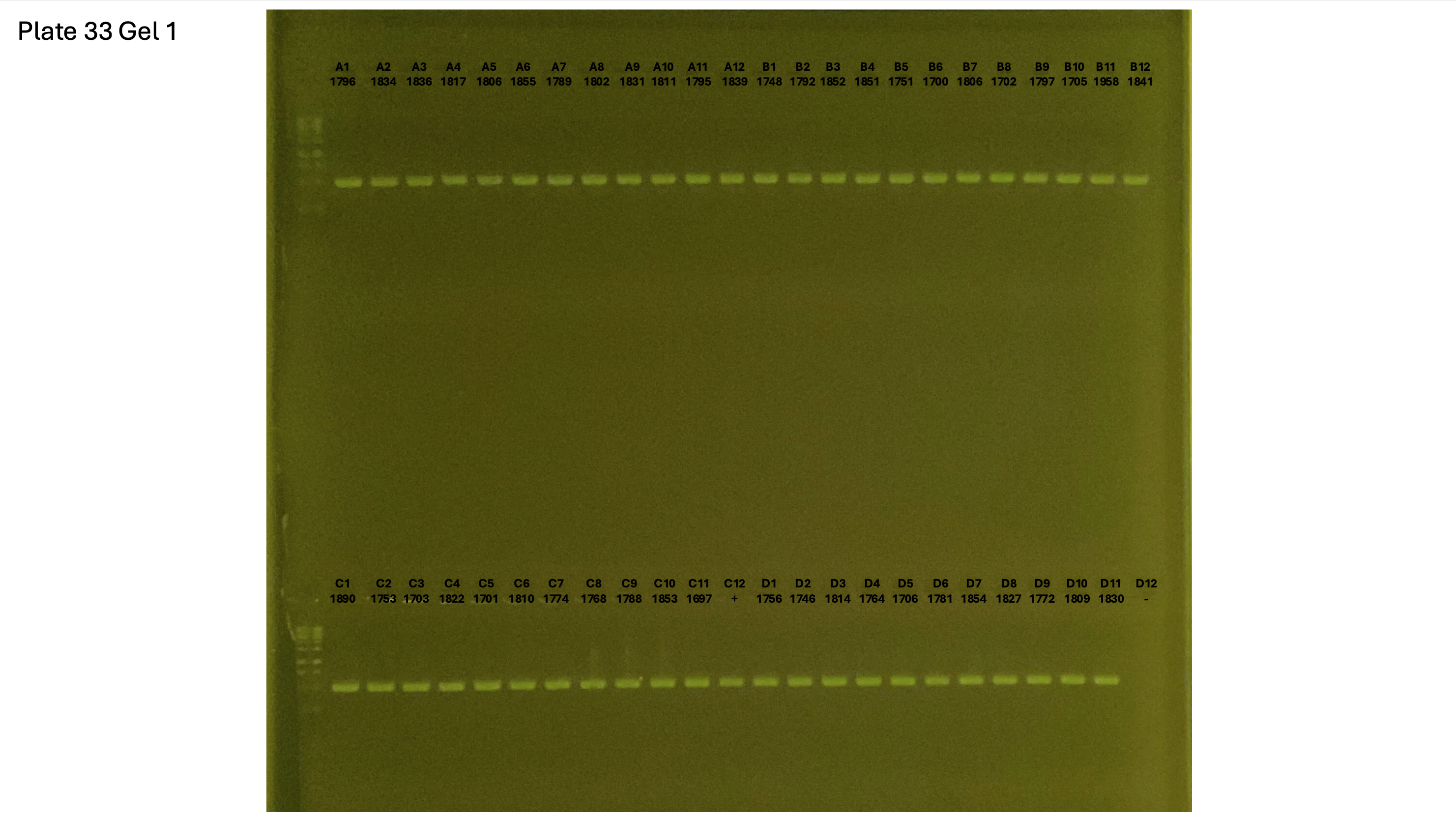
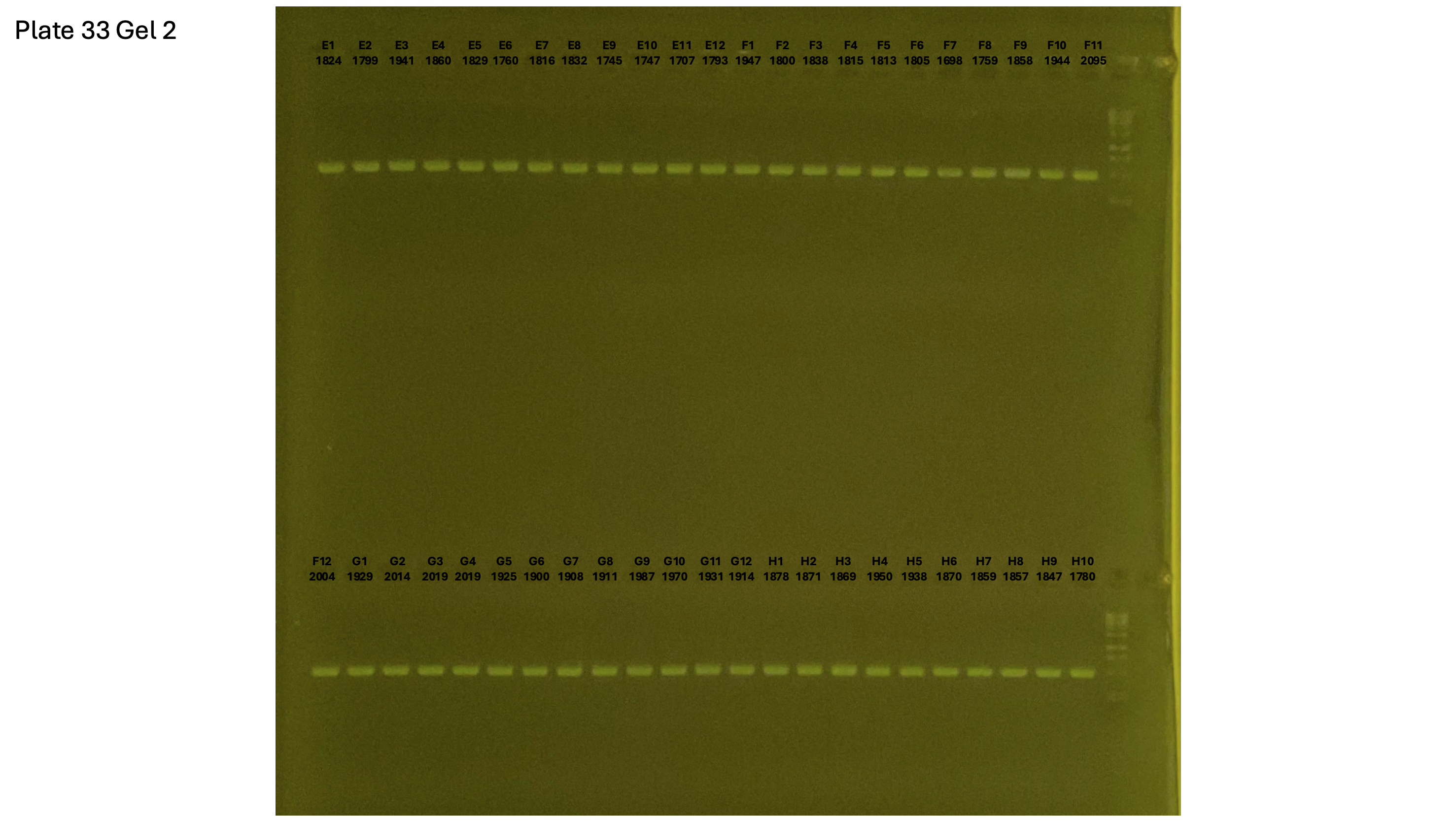
20241002 plate033 gel 001 mtORF after extraction:
20241002 plate033 gel 002 mtORF after extraction:
20241002 plate033 gel 003 mtORF after extraction:
20241002 plate034 gel 001 mtORF after extraction:
20241002 plate034 gel 002 mtORF after extraction:
20241002 plate034 gel 003 mtORF after extraction:
20241002 plate035 gel 001 mtORF after extraction:
20241002 plate035 gel 002 mtORF after extraction:
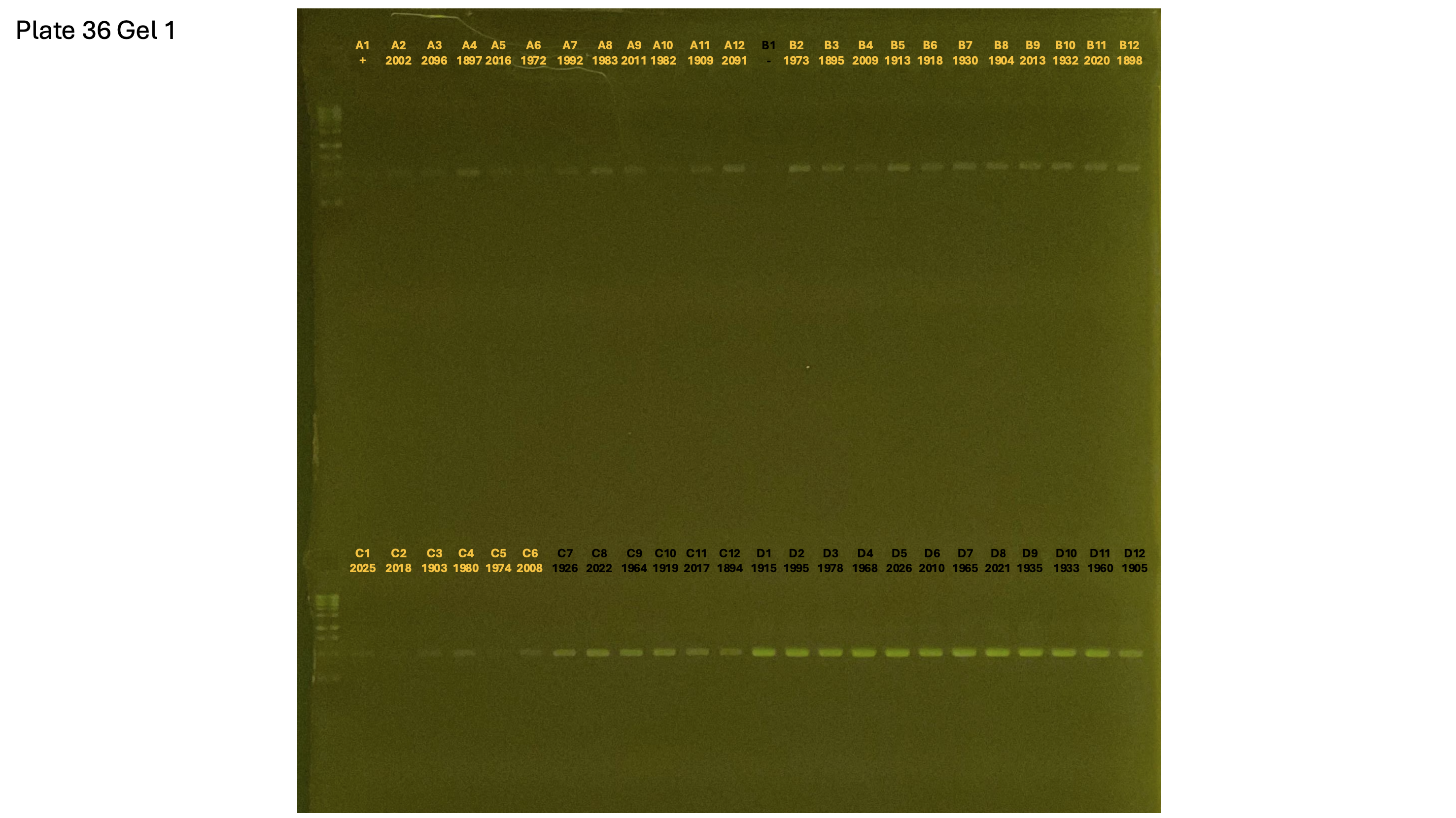
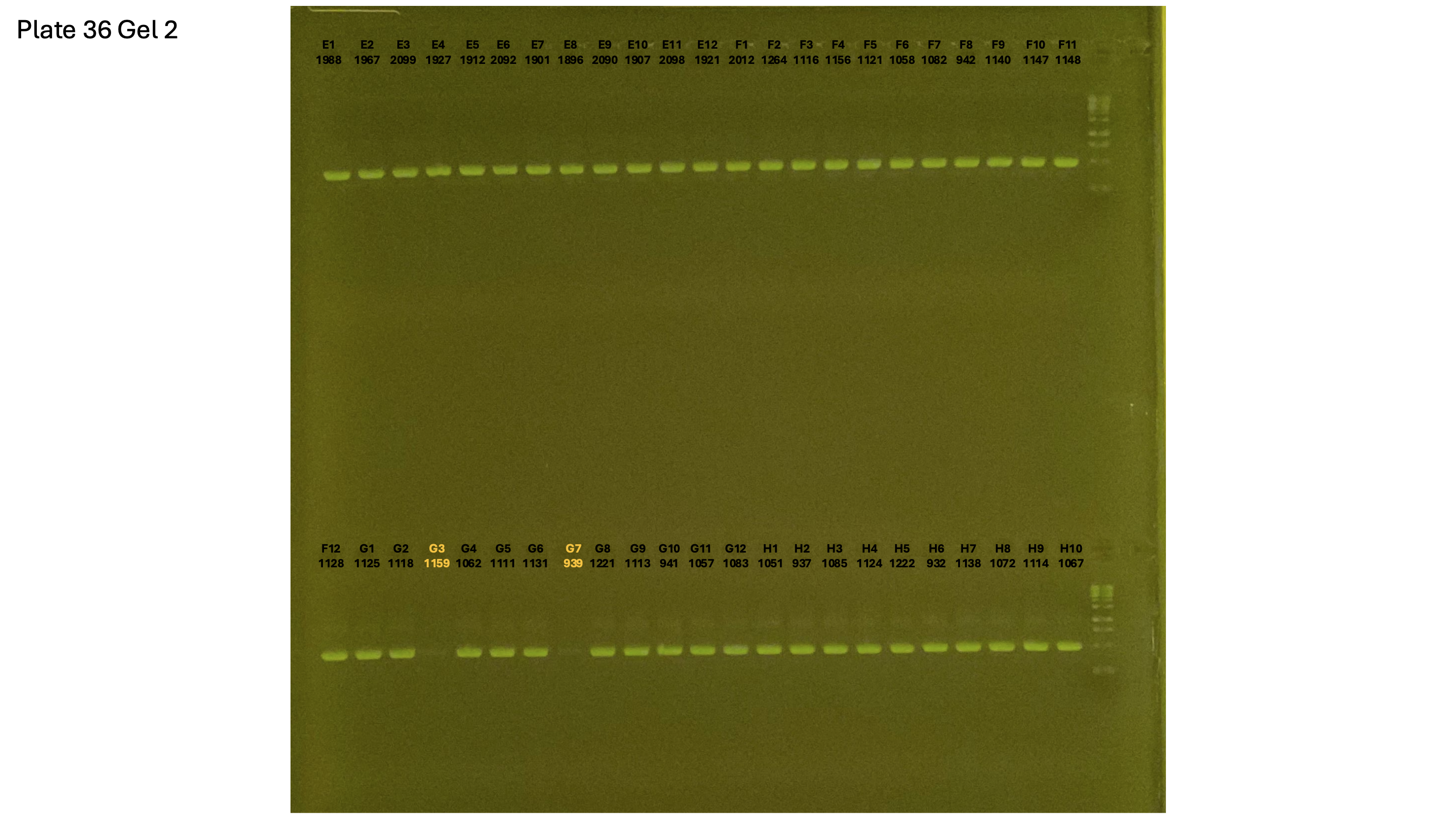
20241002 plate036 gel 001 mtORF after extraction:
20241002 plate036 gel 002 mtORF after extraction:
20241002 plate036 gel 003 mtORF after extraction:
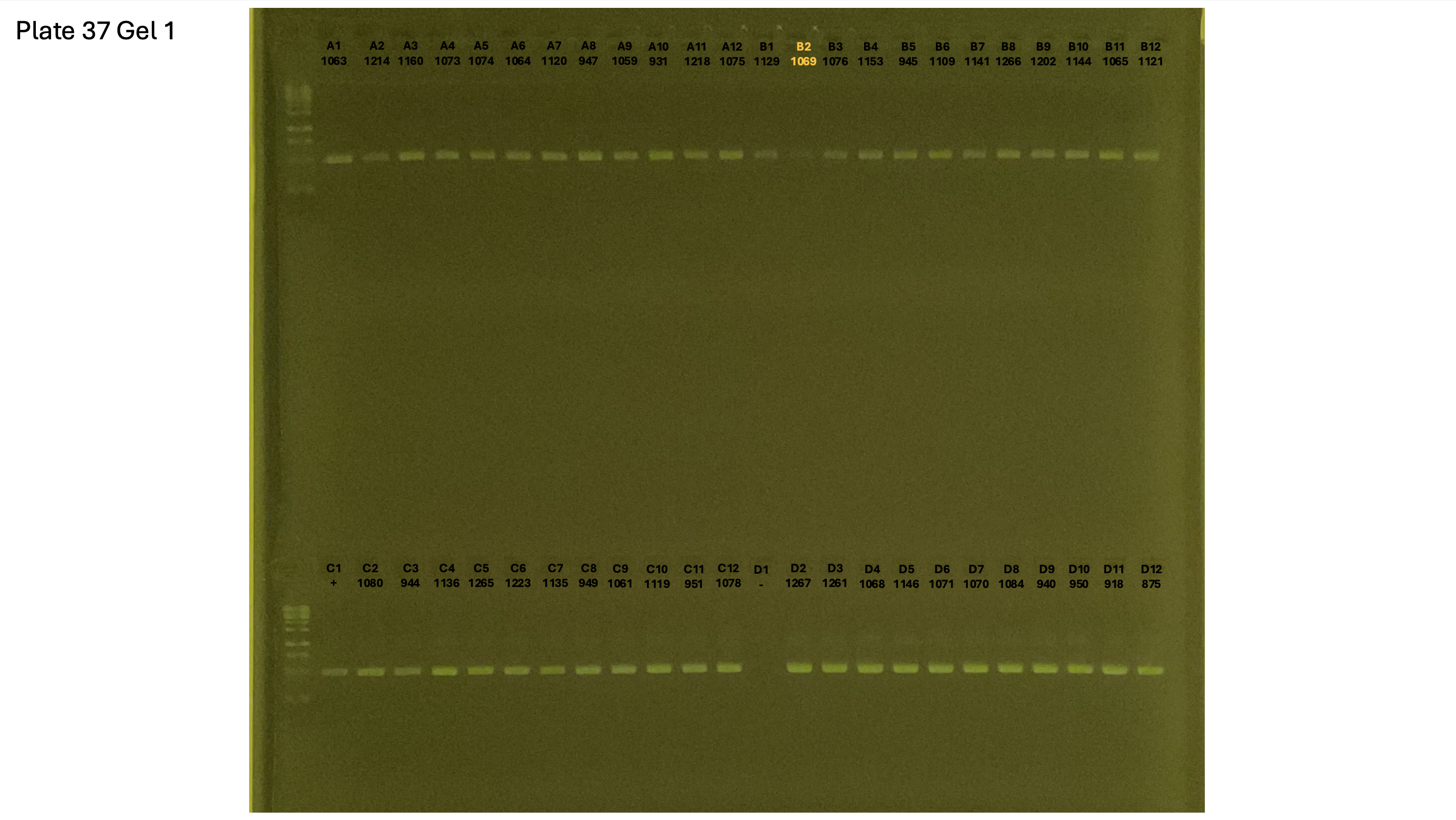
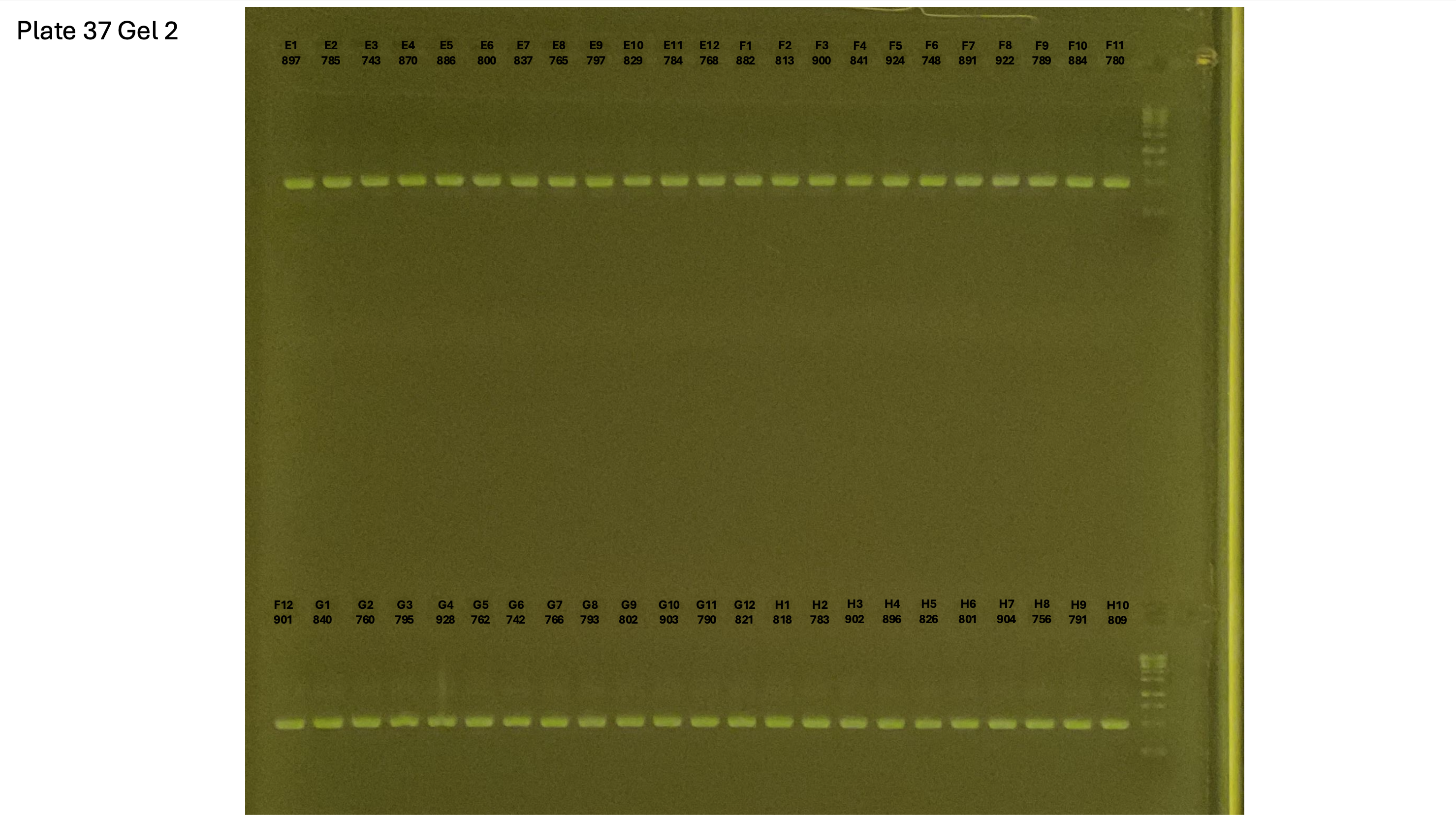
20241002 plate037 gel 001 mtORF after extraction:
20241002 plate037 gel 002 mtORF after extraction:
20241002 plate037 gel 003 mtORF after extraction:
20241002 plate038 gel 001 mtORF after extraction:
20241002 plate038 gel 002 mtORF after extraction:
20241002 plate038 gel 003 mtORF after extraction:
20241002 plate039 gel 001 mtORF after extraction:
20241002 plate039 gel 002 mtORF after extraction:
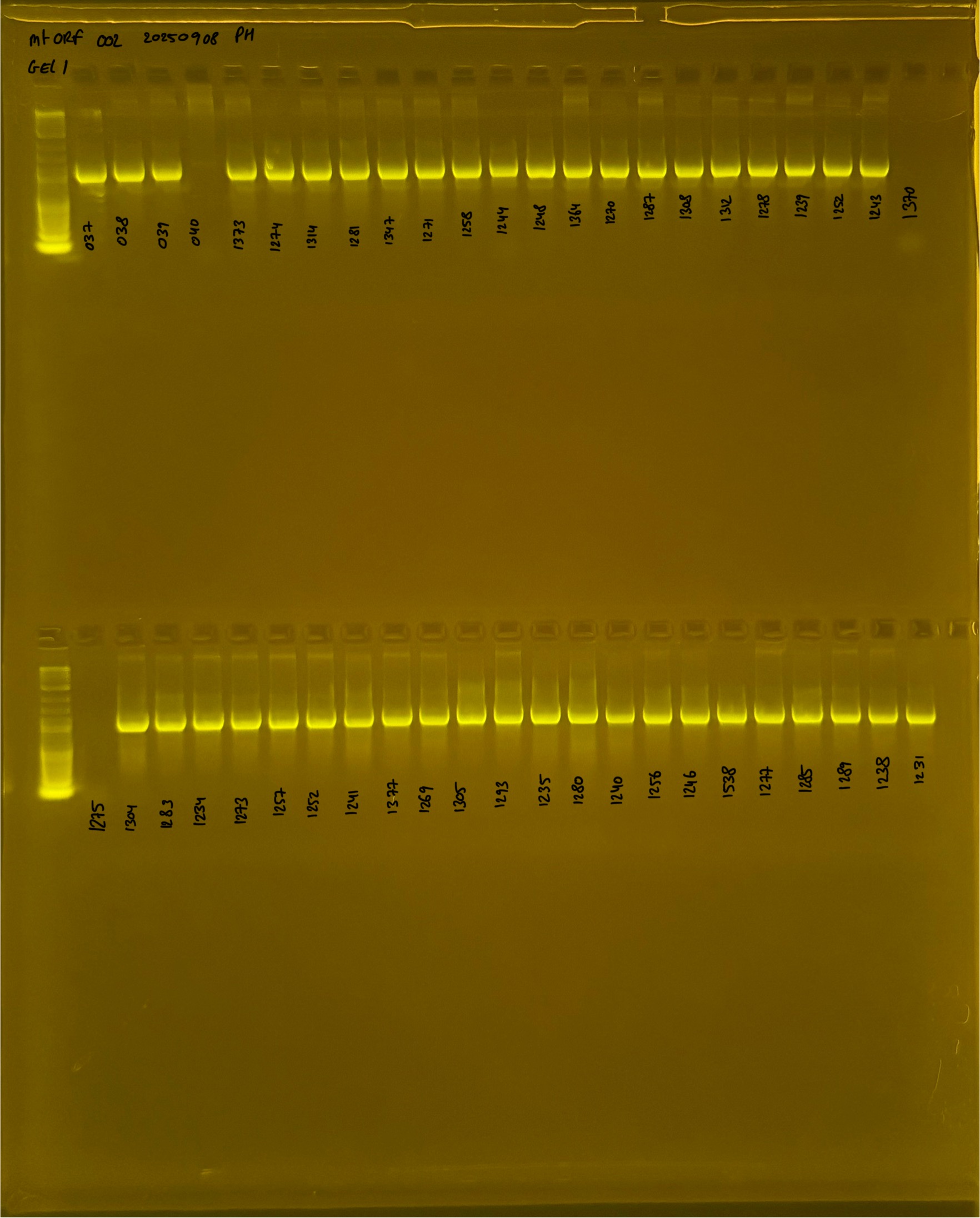
20250908 mtORF 002 gel 1 for resenquence the plate:
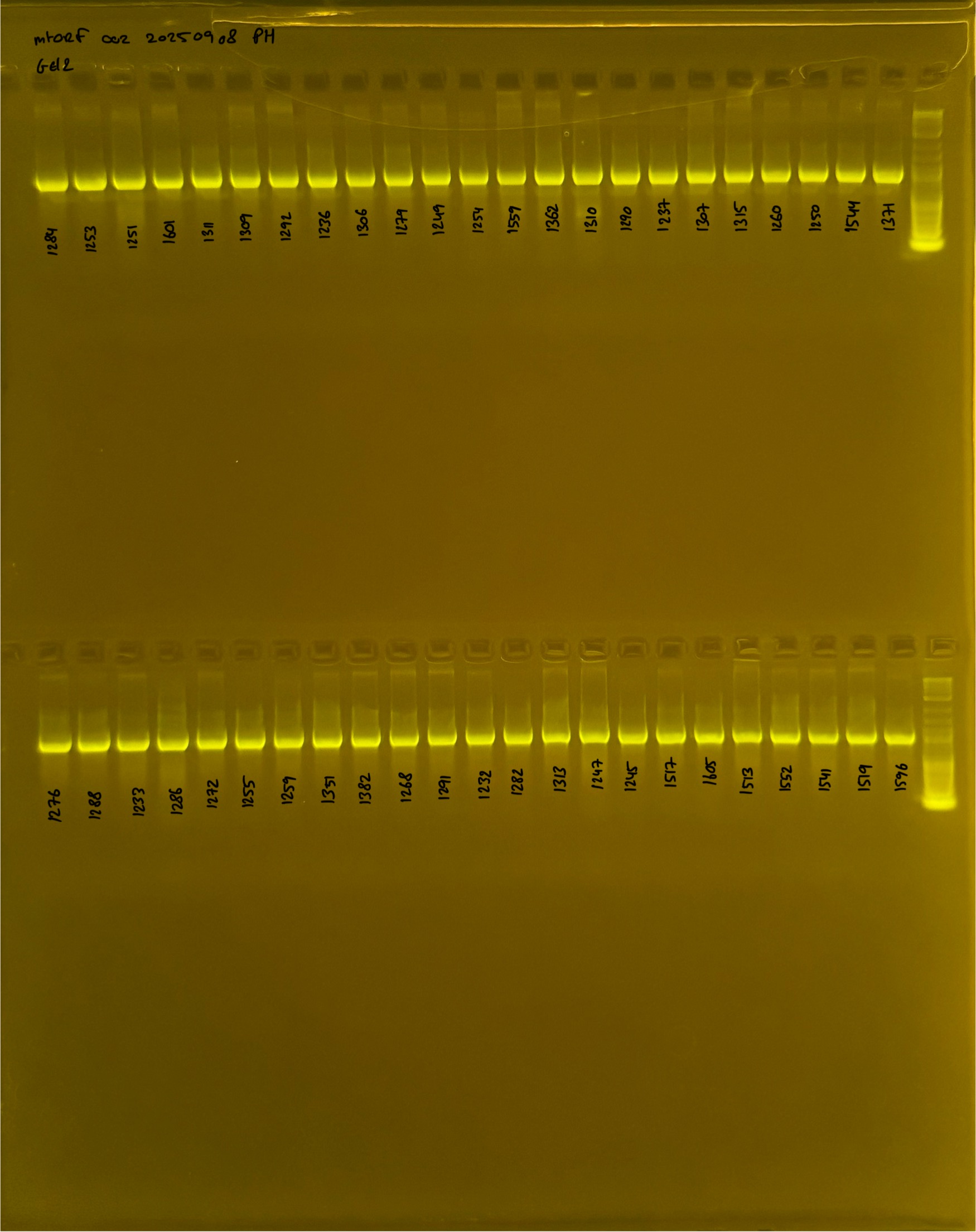
20250908 mtORF 002 gel 2 for resenquence the plate:
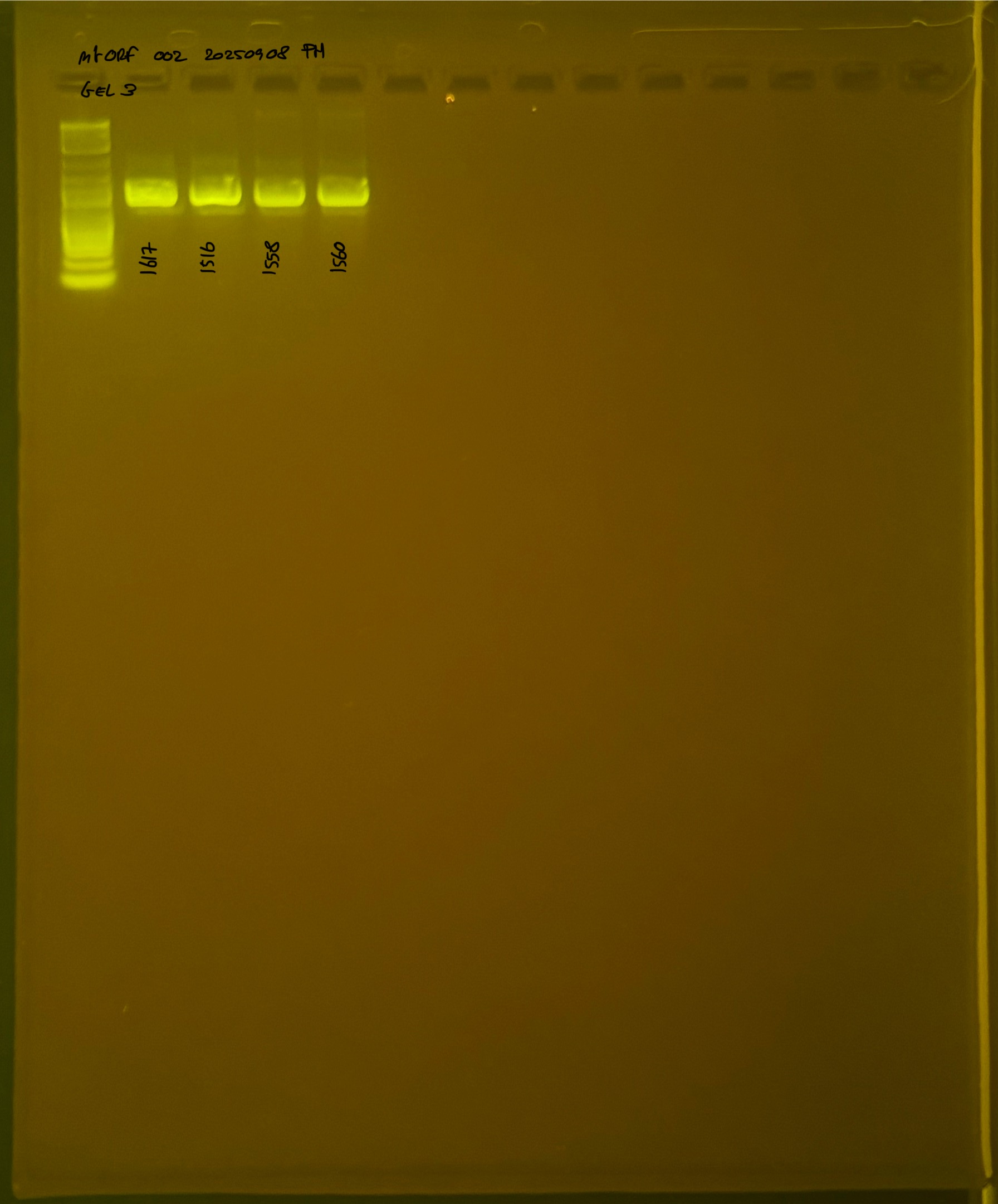
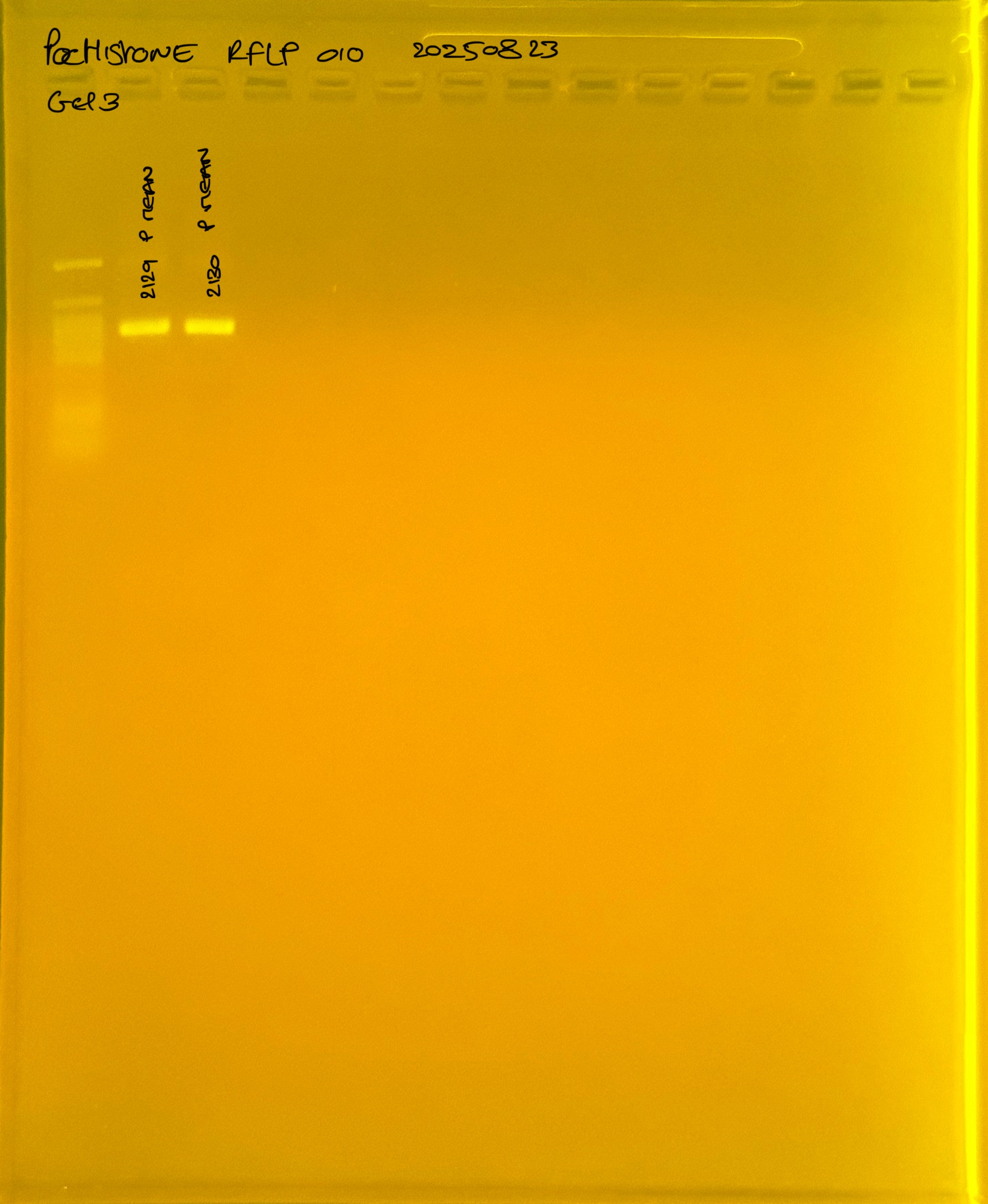
20250908 mtORF 002 gel 3 for resenquence the plate:
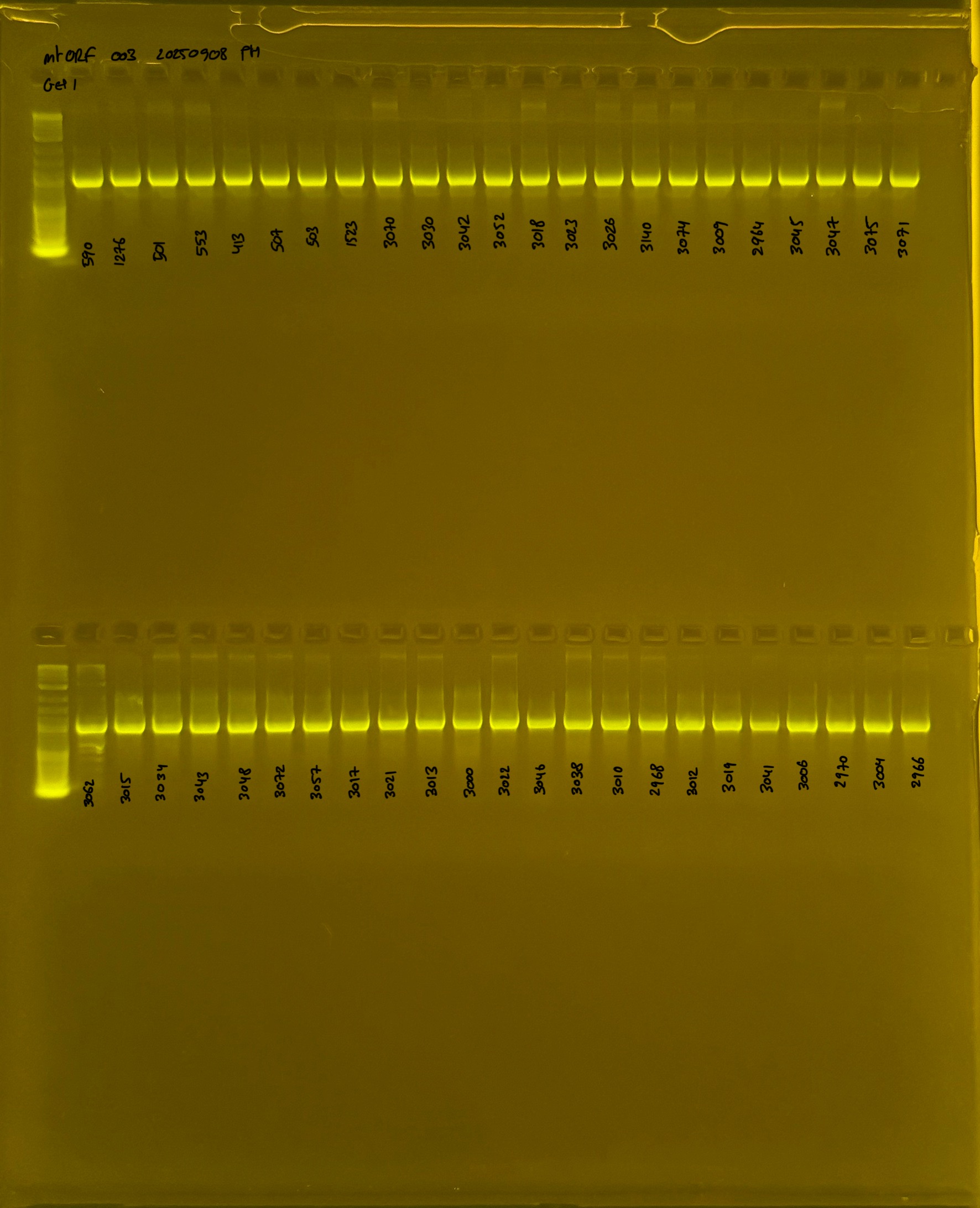
20250908 mtORF 003 gel 1 for resenquence the plate:
20250908 mtORF 003 gel 2 for resenquence the plate:
20250908 mtORF 003 gel 3 for resenquence the plate:
ZR 96 DNA Clean-up Kit Cat No D4017 D4018
Protocol:
-
Reagent preparation: Add 96 ml 100% ethanol (104 ml 95% ethanol) to the 24 ml DNA Wash Buffer concentrate. Add 192 ml 100% ethanol (208 ml 95% ethanol) to the 48ml DNA Wash Buffer concentrate.
-
Sample processing:
-
Add 100 µl DNA Binding Buffer to PCR sample, Mix, transfer to cullum plate, then add any remaning sample (20µl).
-
Centrifuge at 3000g for 5 minutes
-
Add 300 µl Wash buffer to each well
-
Centrifuge at 3000g for 5 minutes
-
Add 300 µl at Wash buffer
-
Centrifuge at 3000g for 5 minutes
-
Add 40µl nuc free water to each well
-
Transfer silicon plate into an evolution plate
-
Centrifuge 3000g for 3 minutes
-
Done! Store in freezer
Sequence process at URI
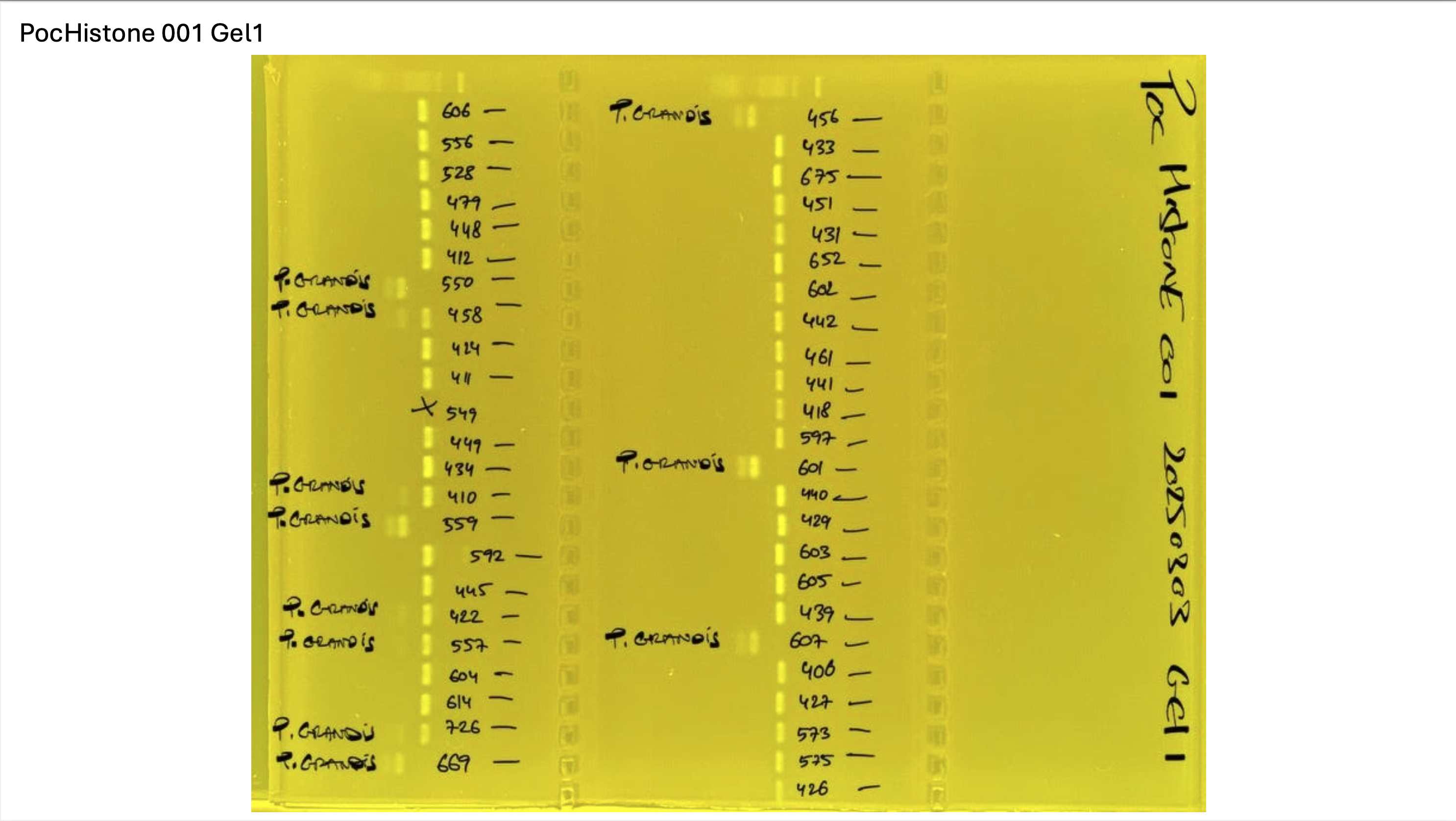
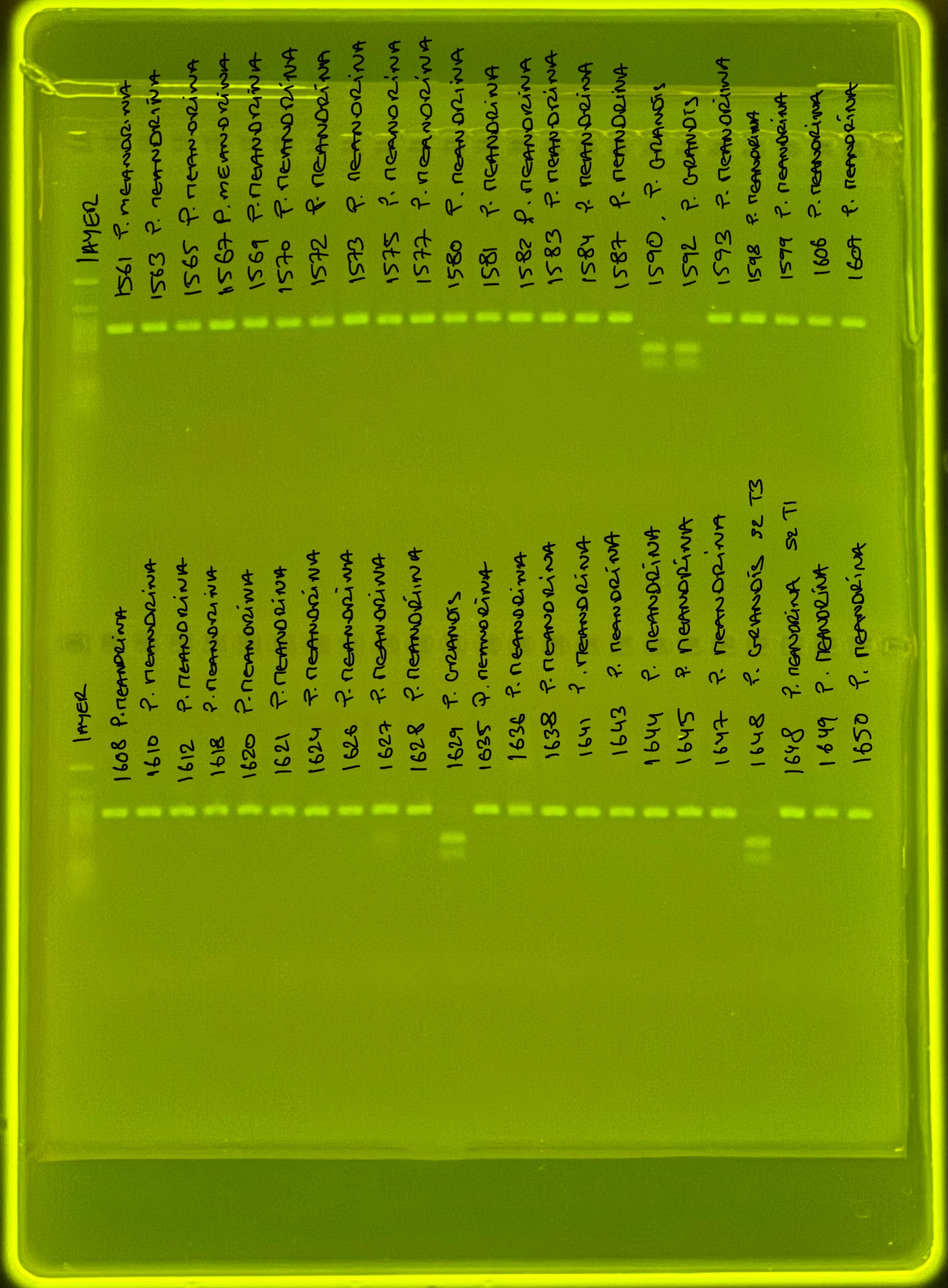
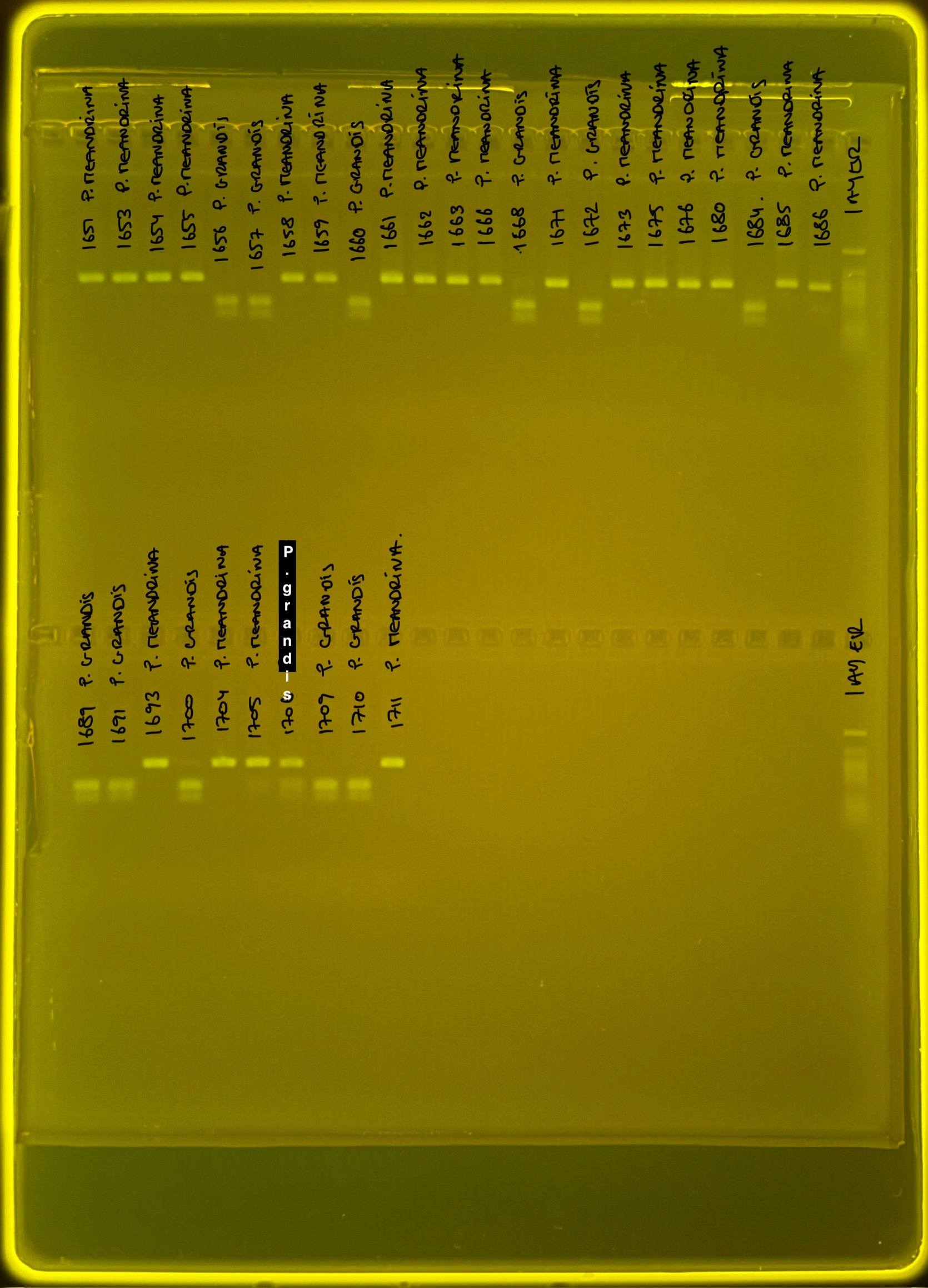
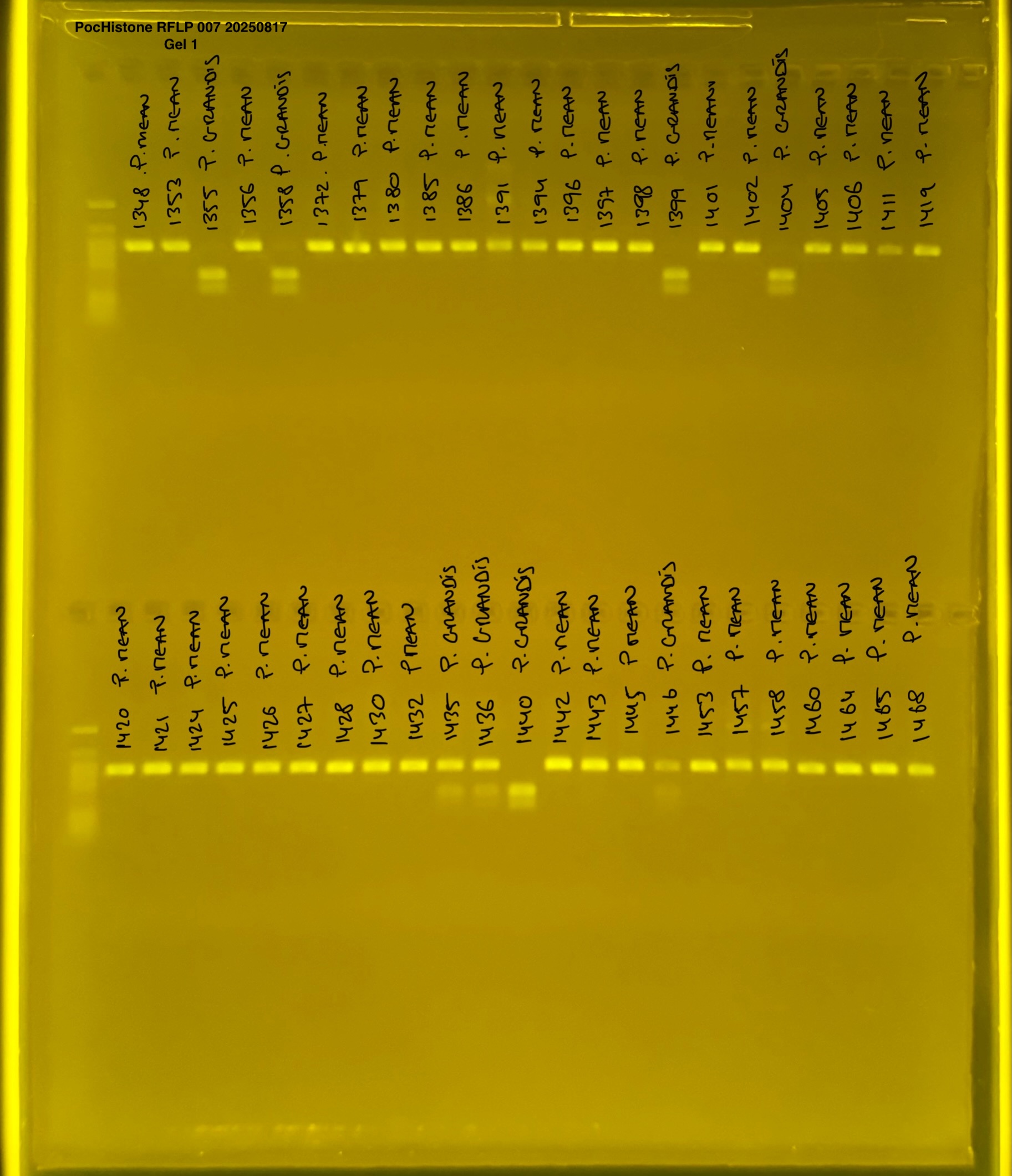
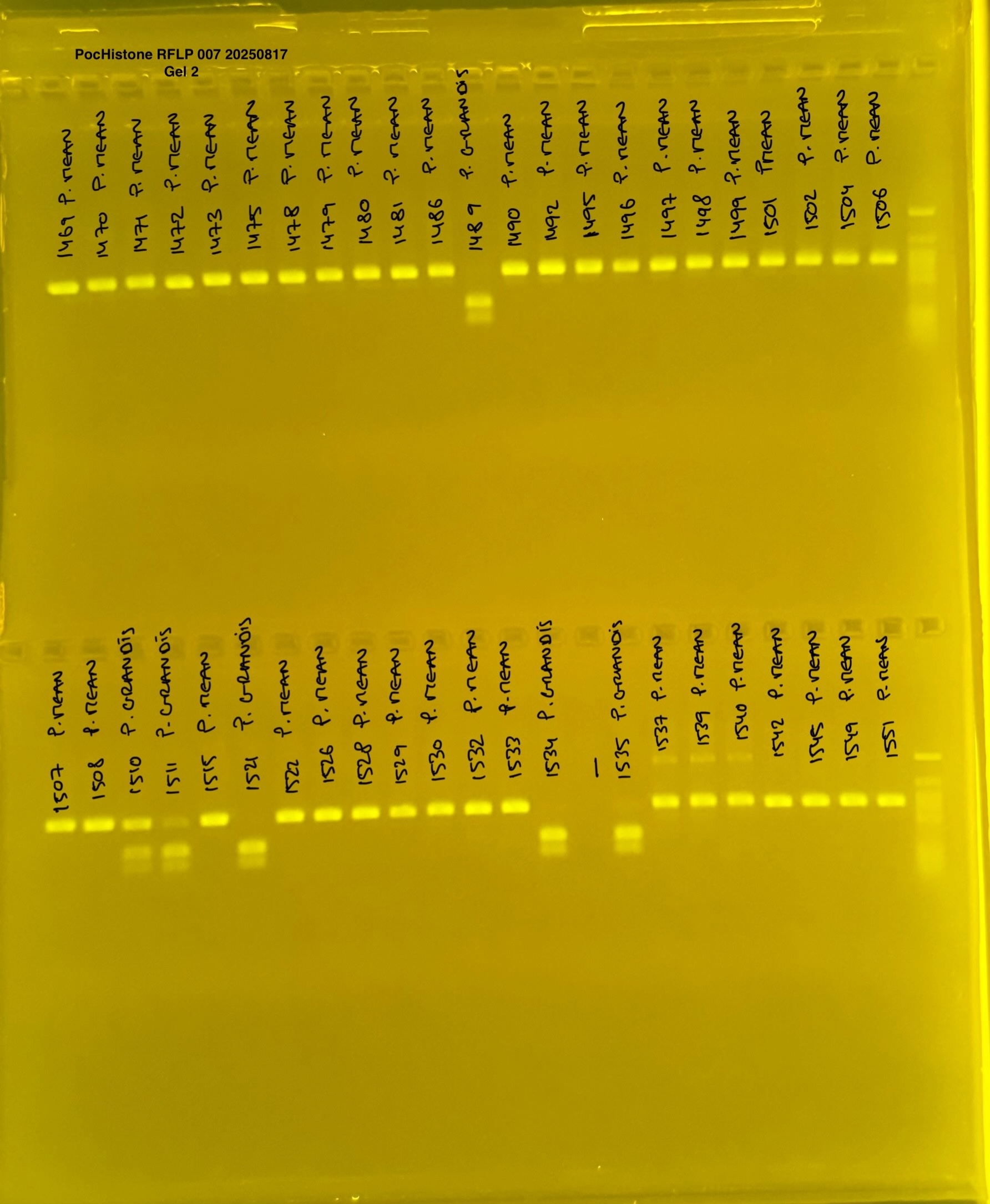
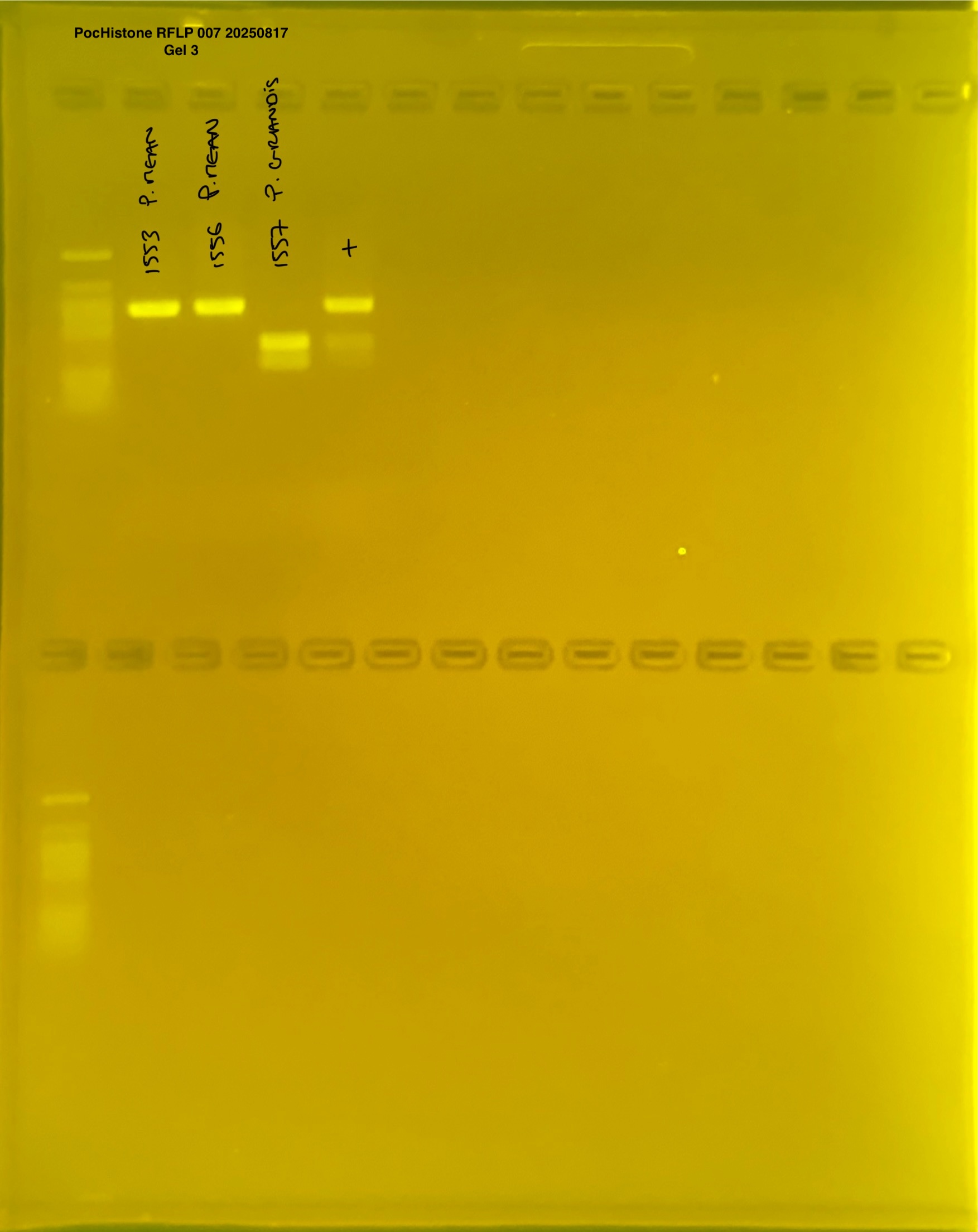
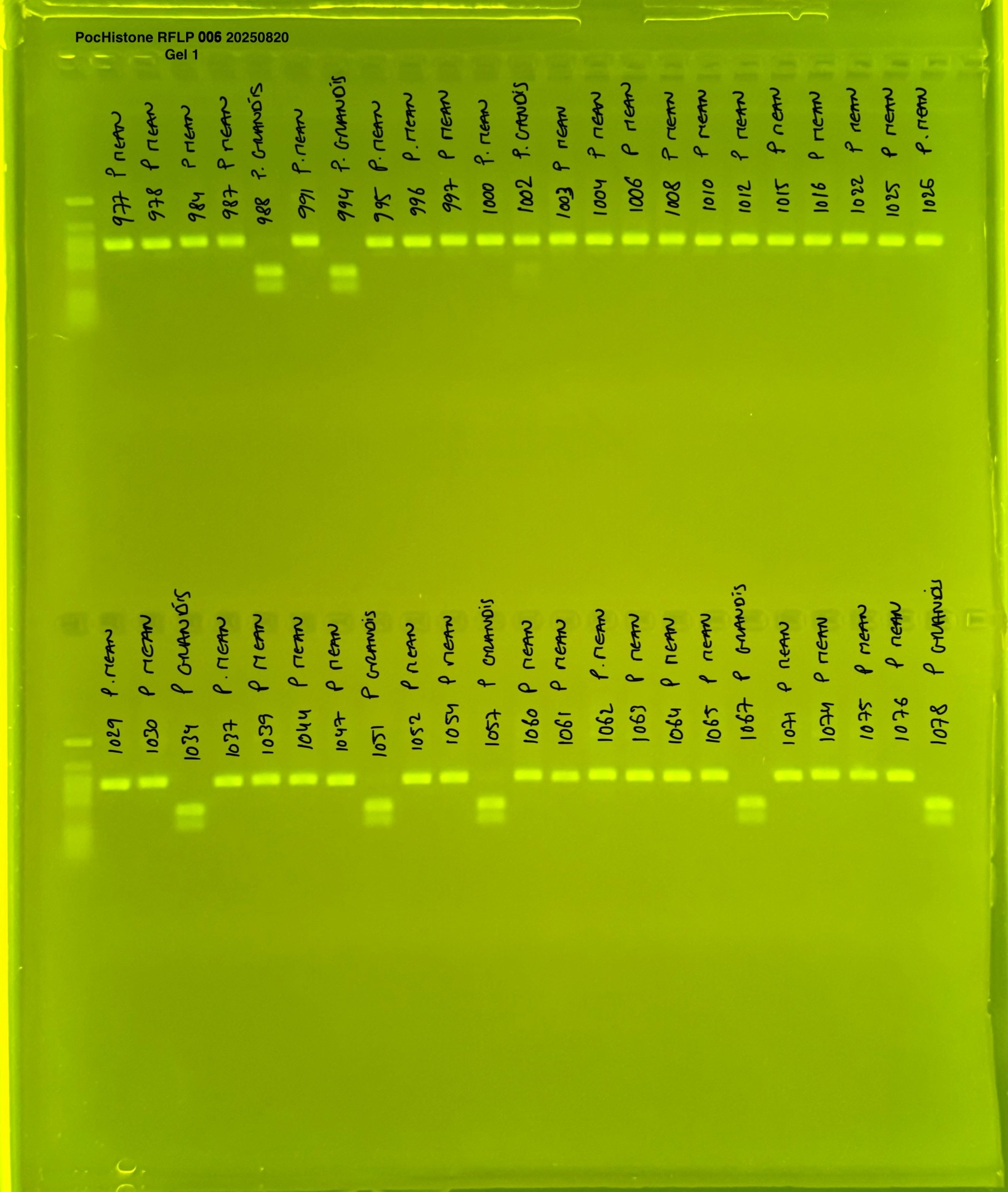
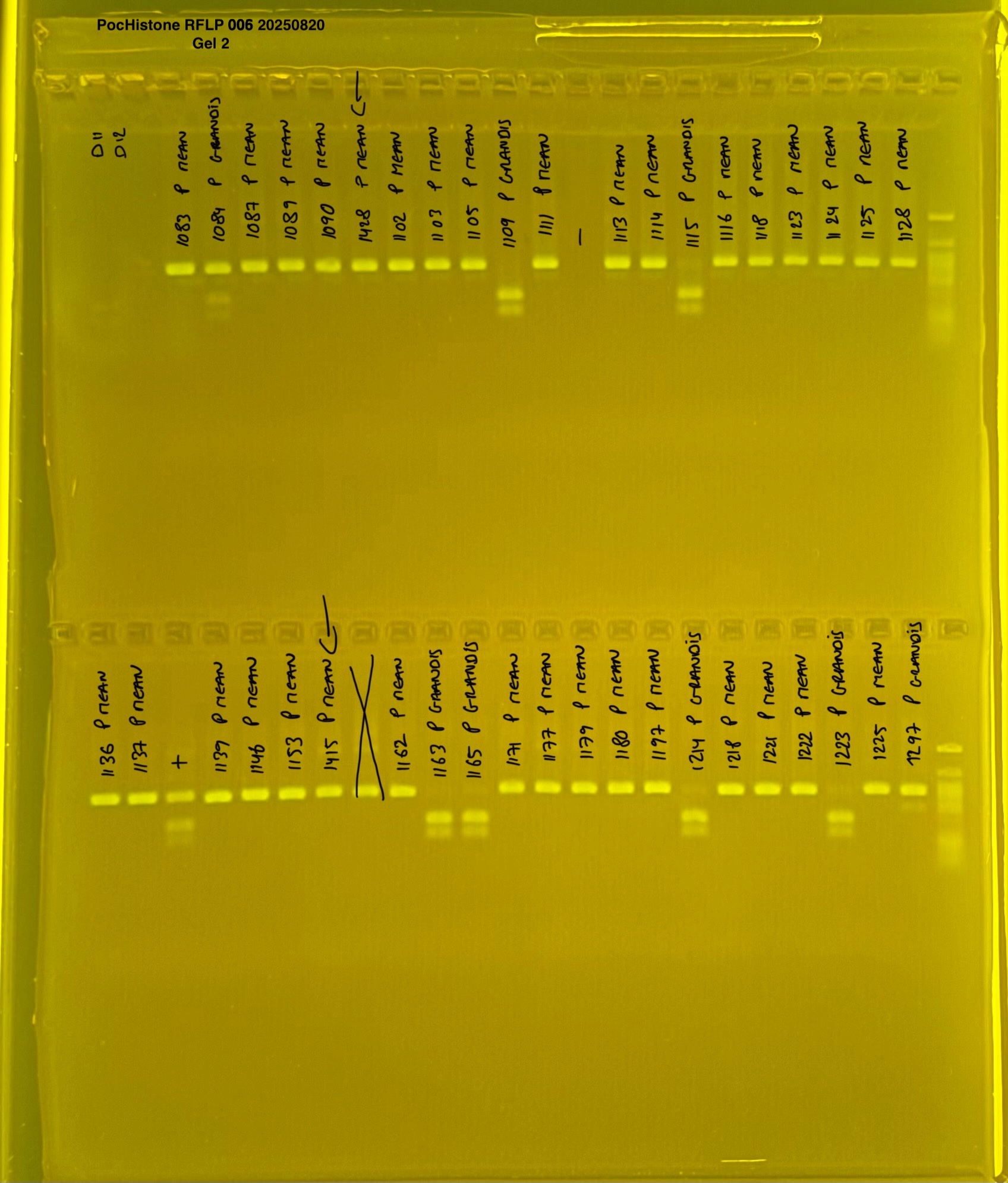
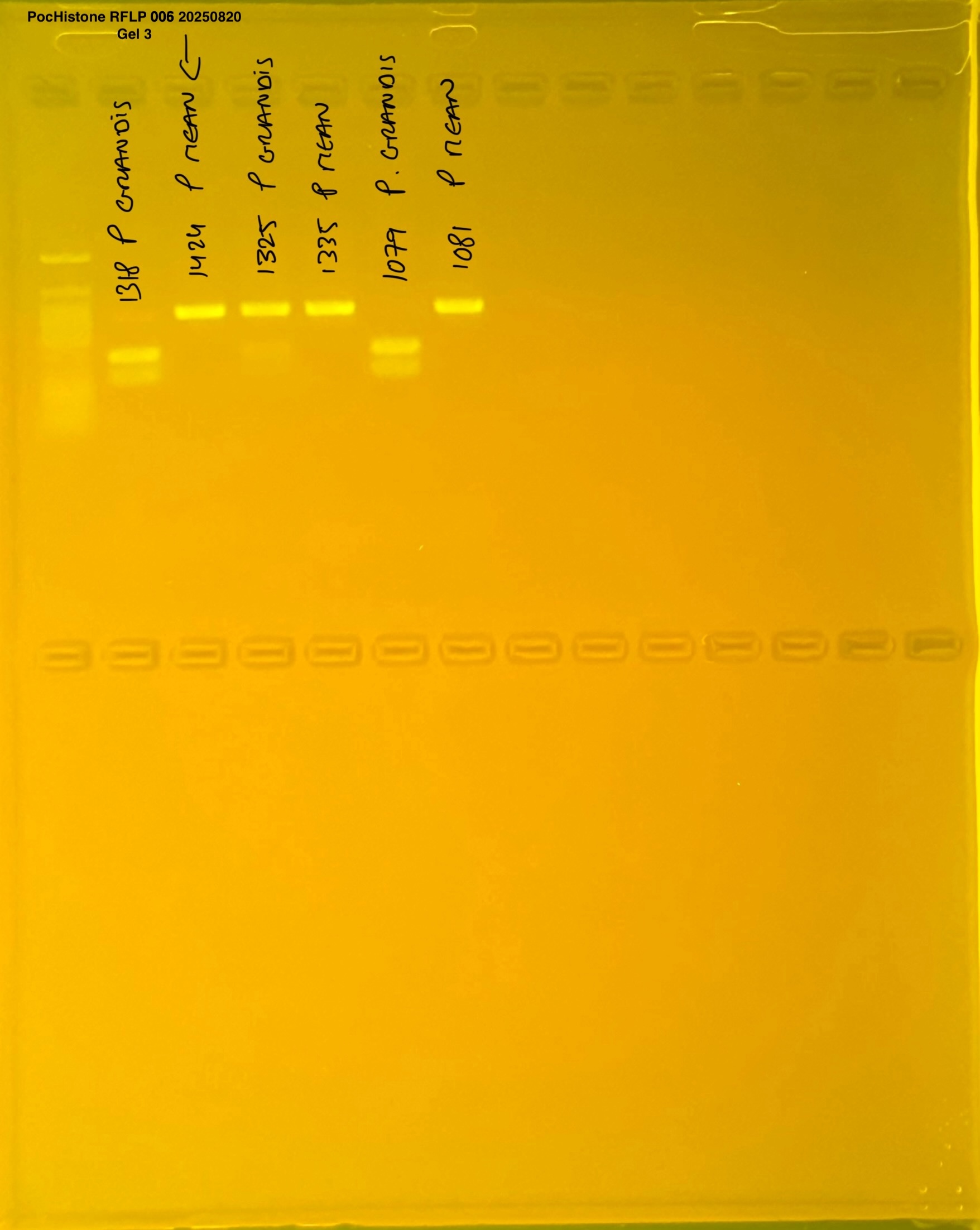
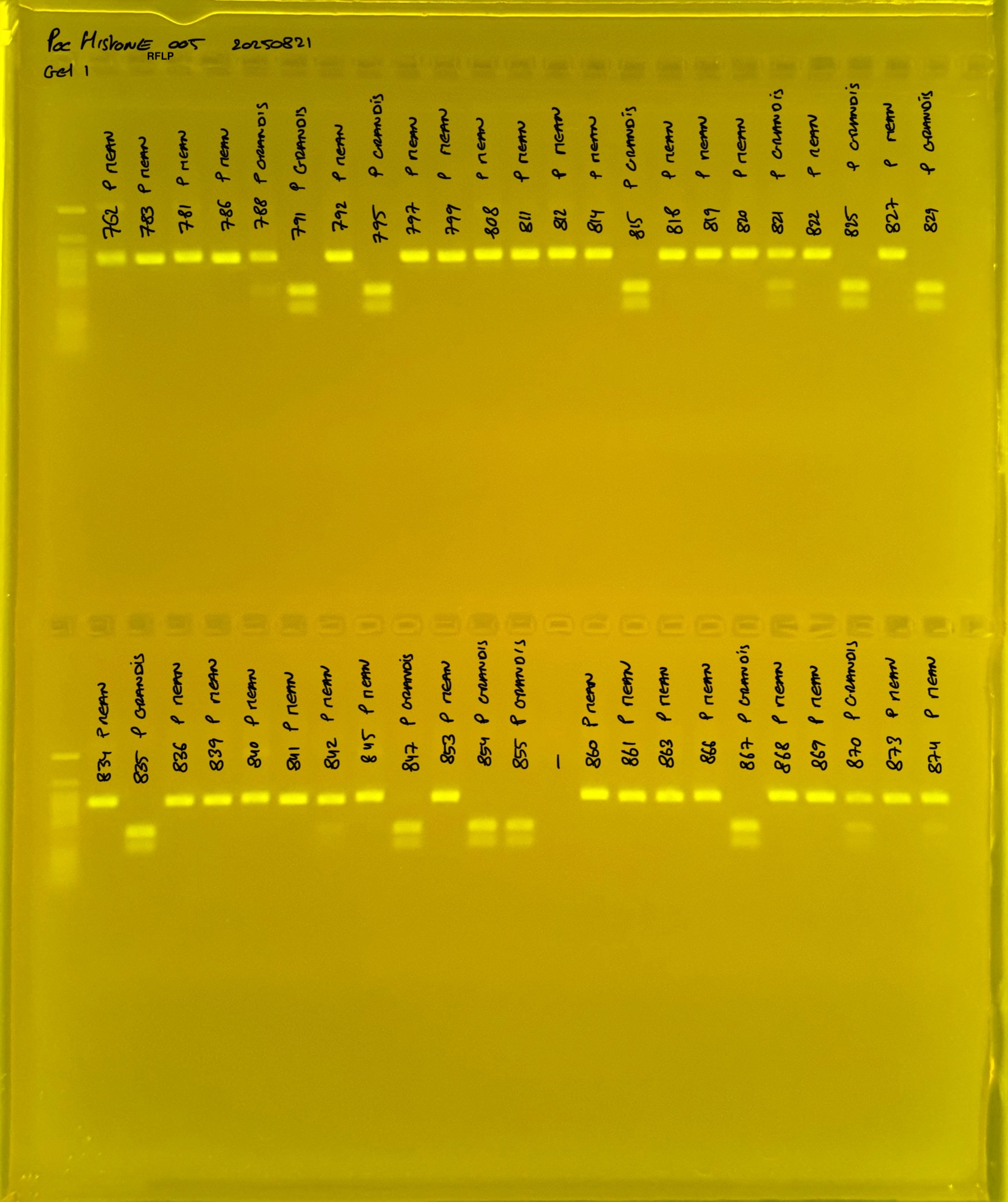
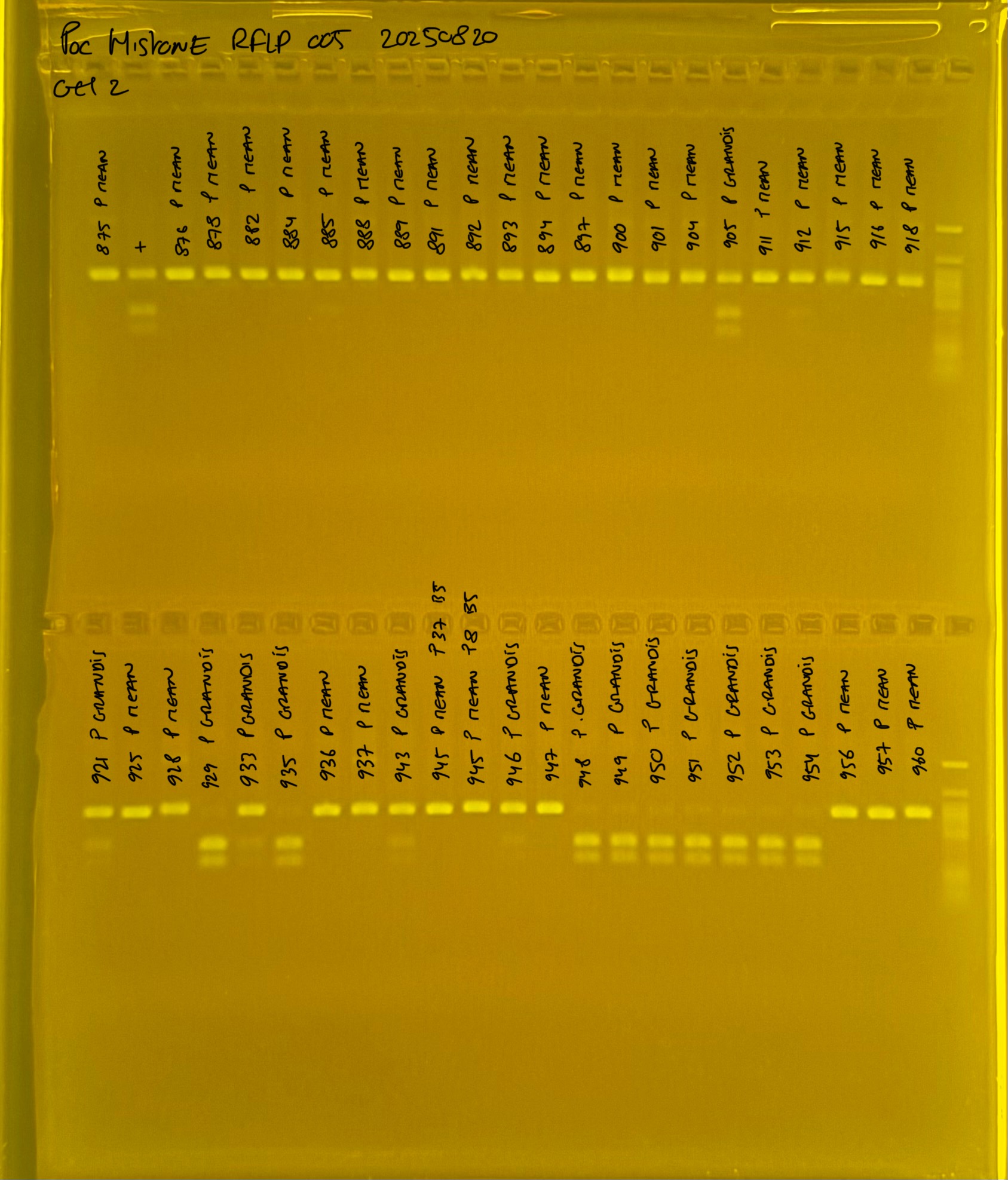
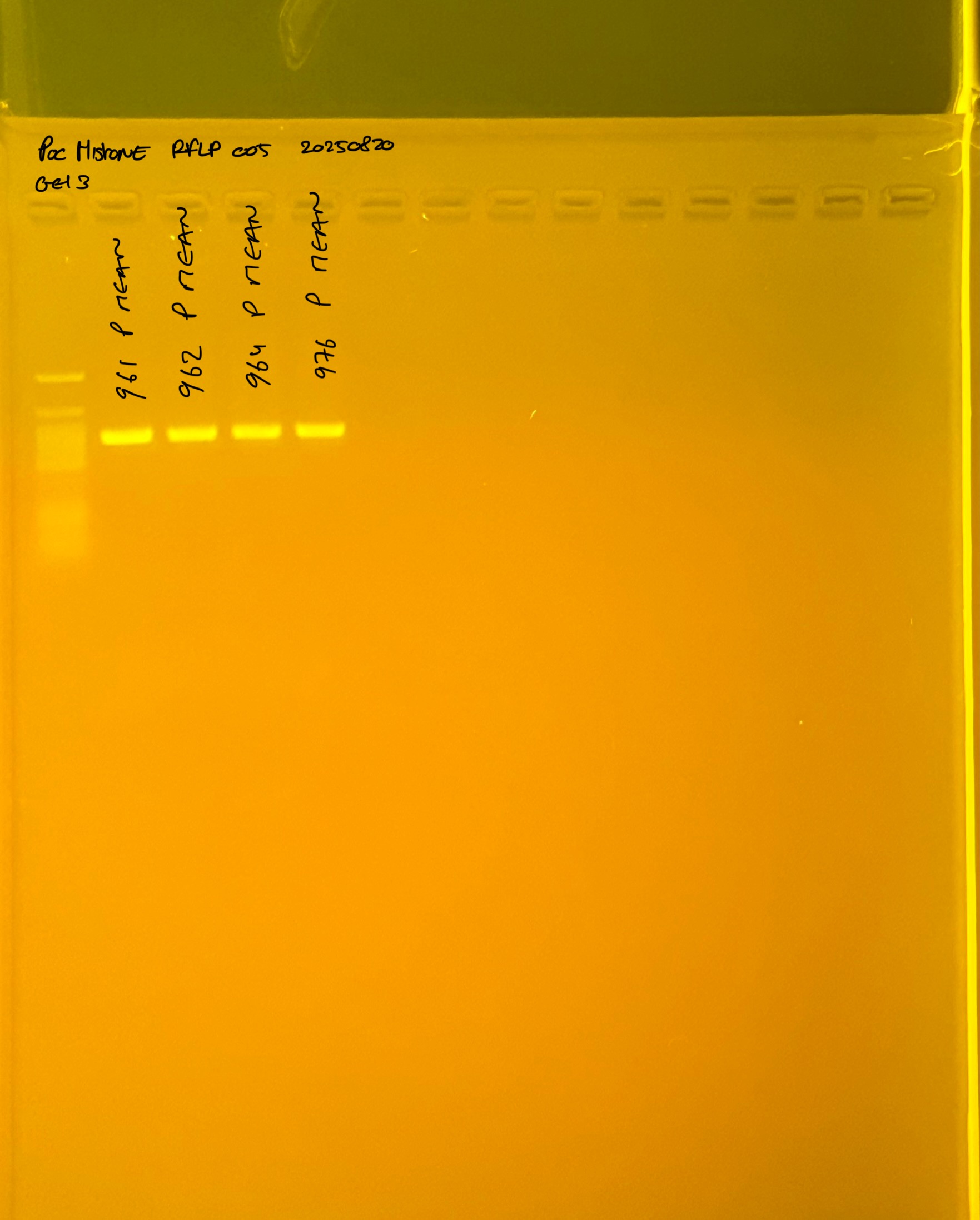
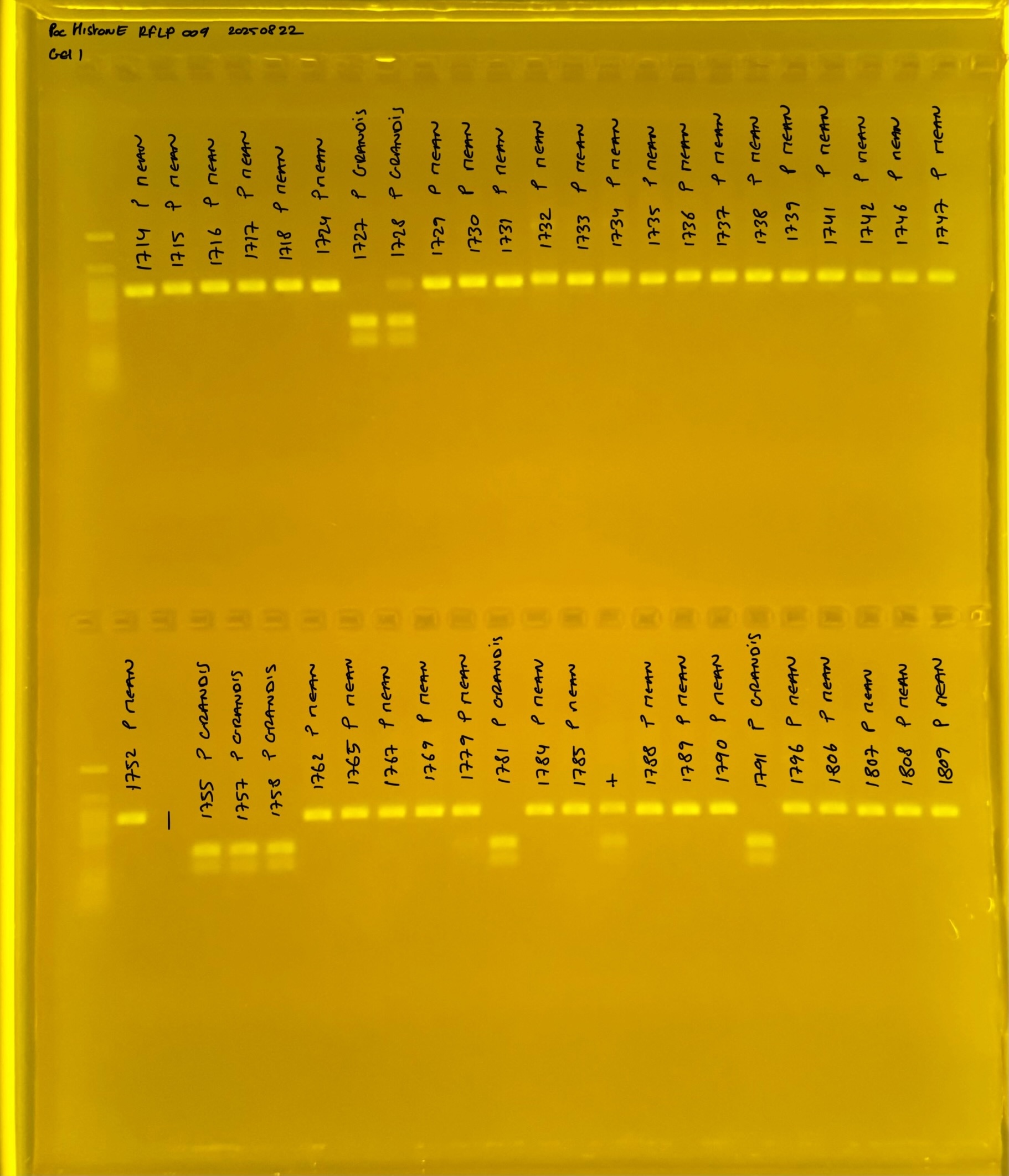
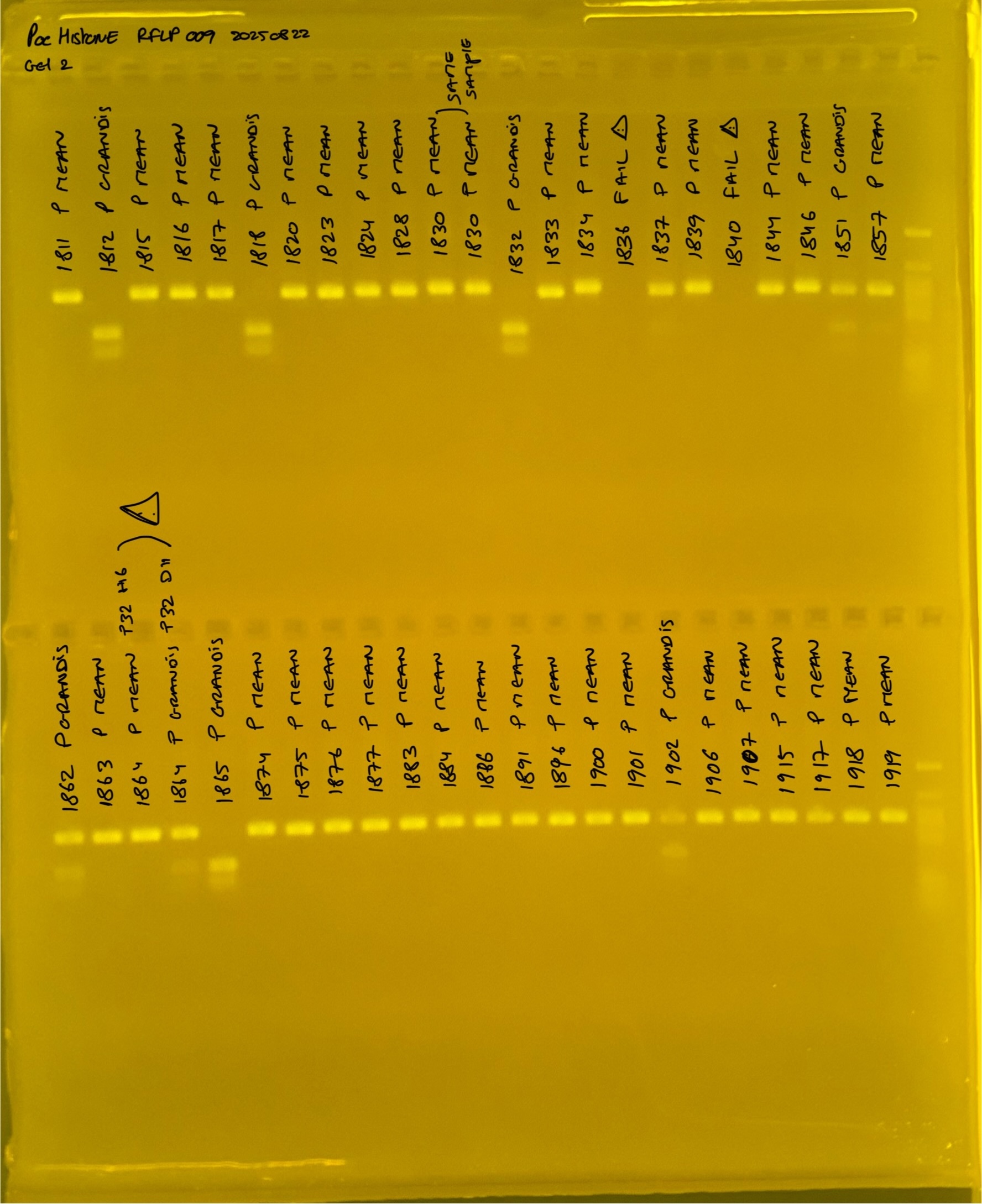
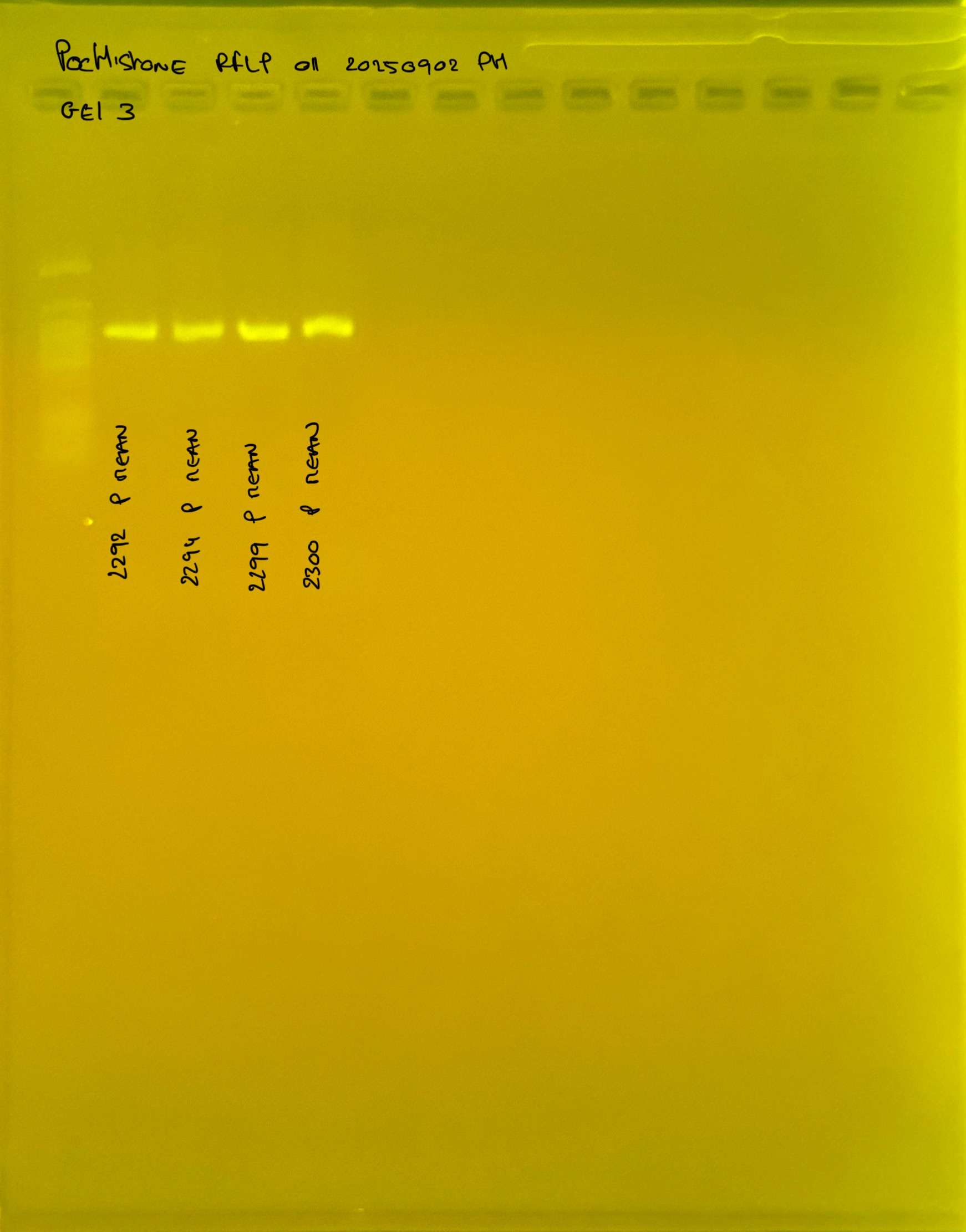
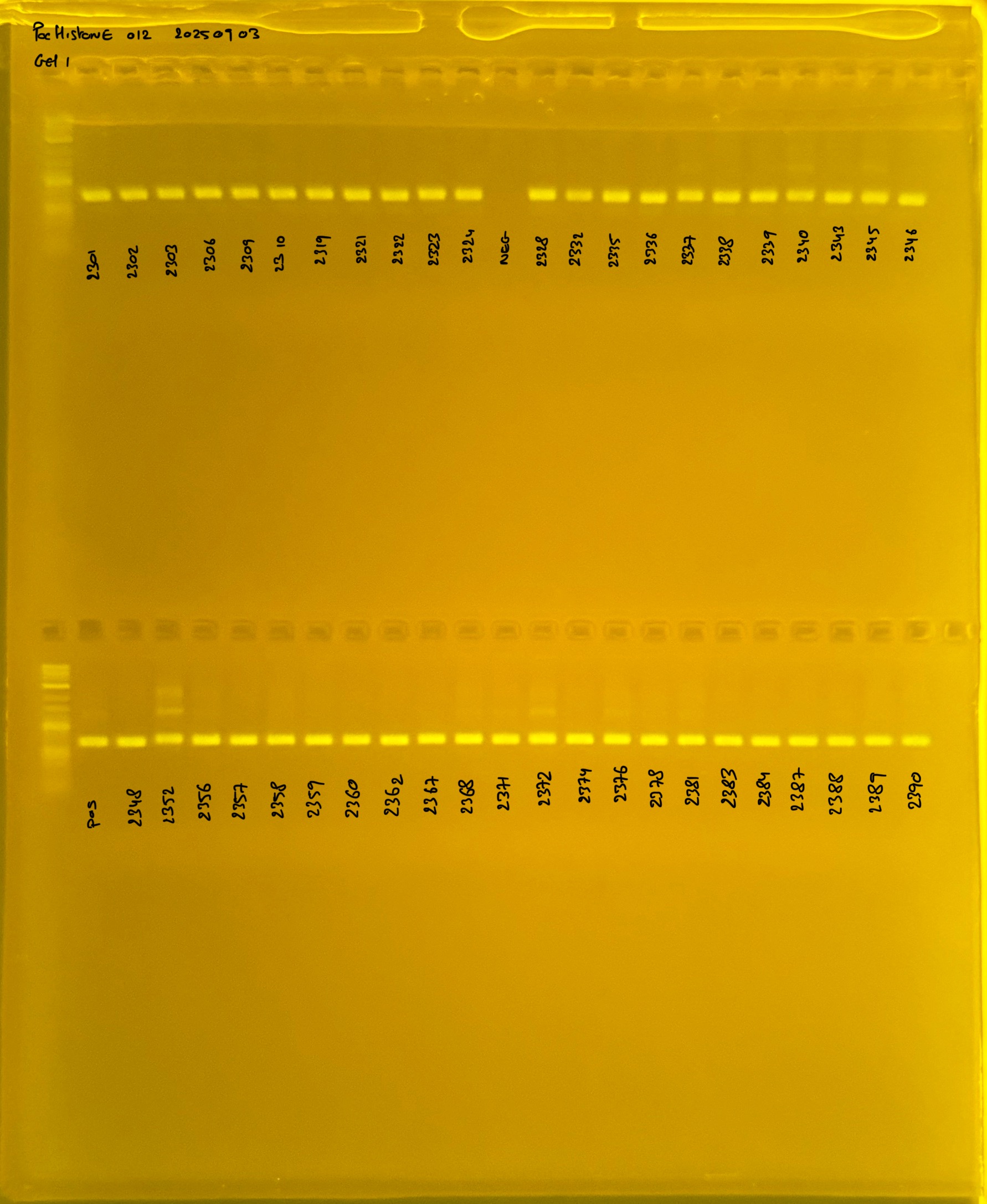
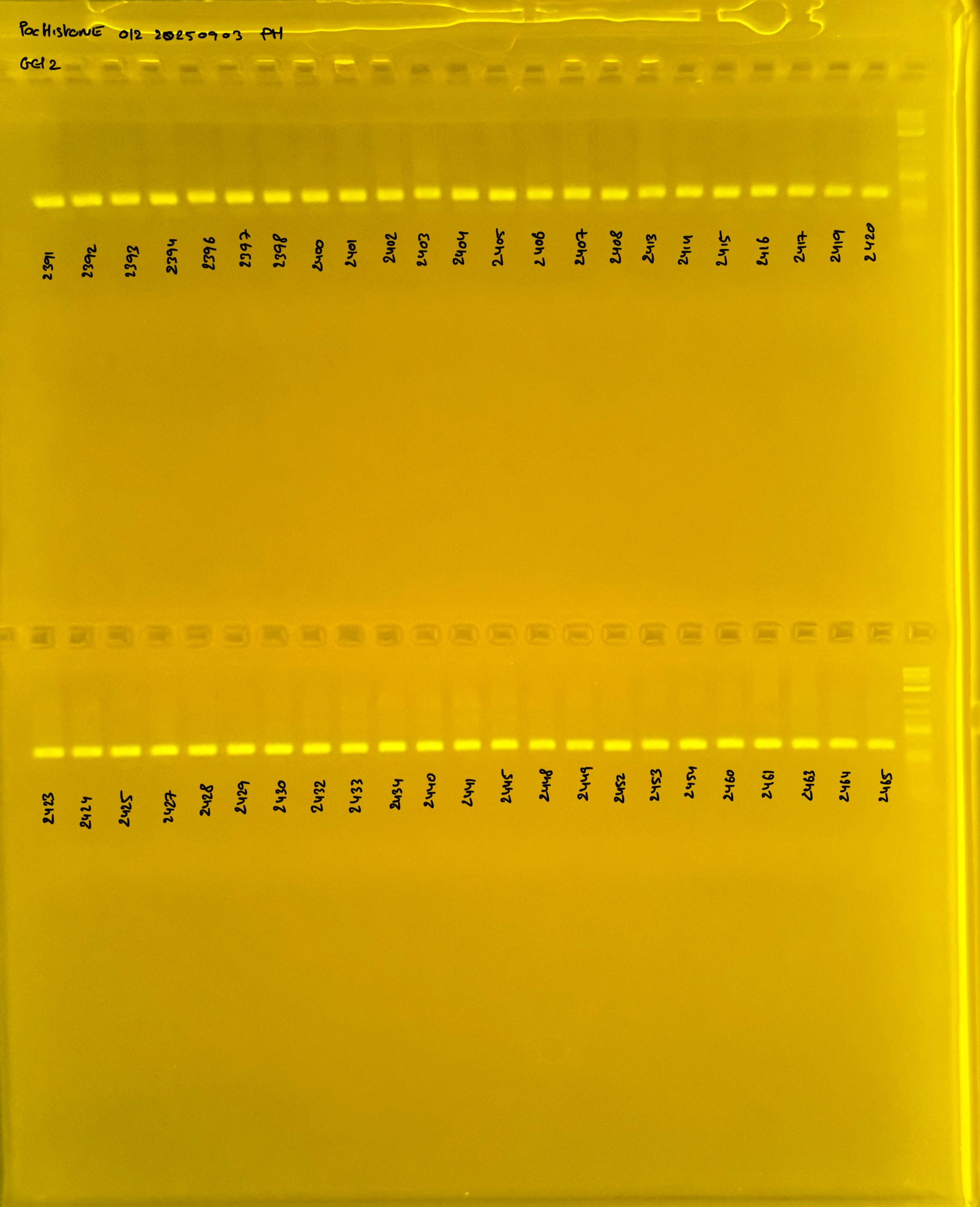
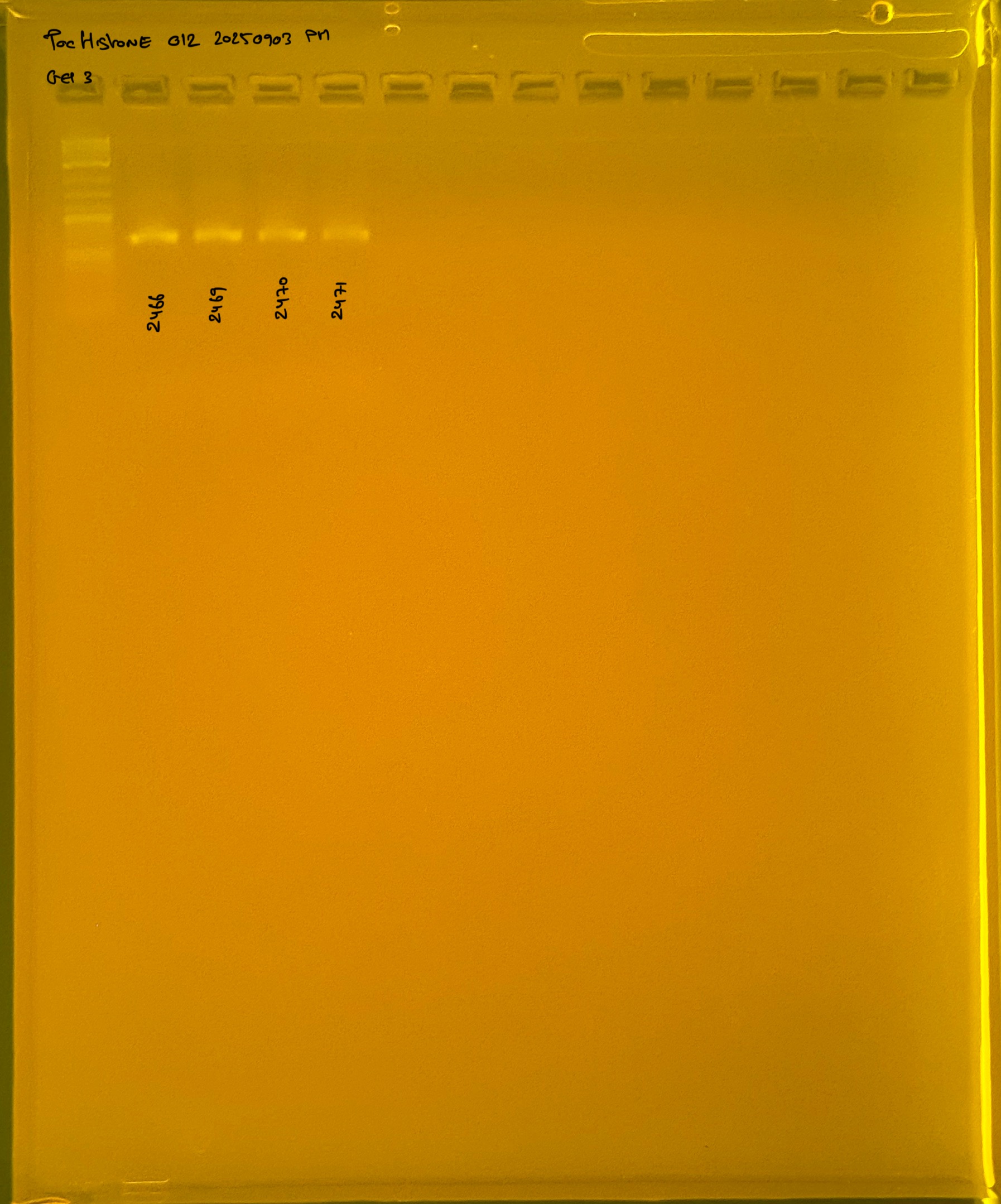
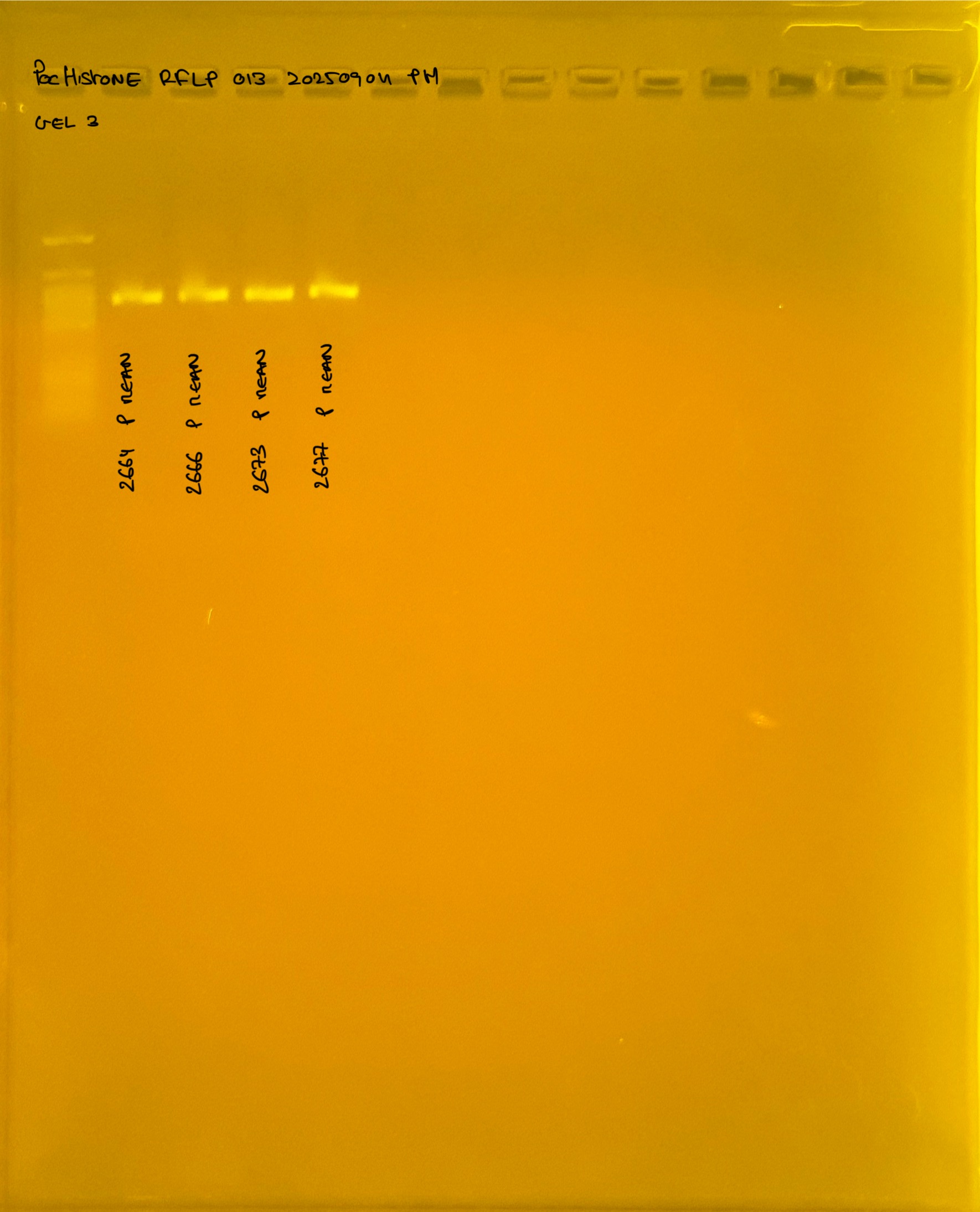
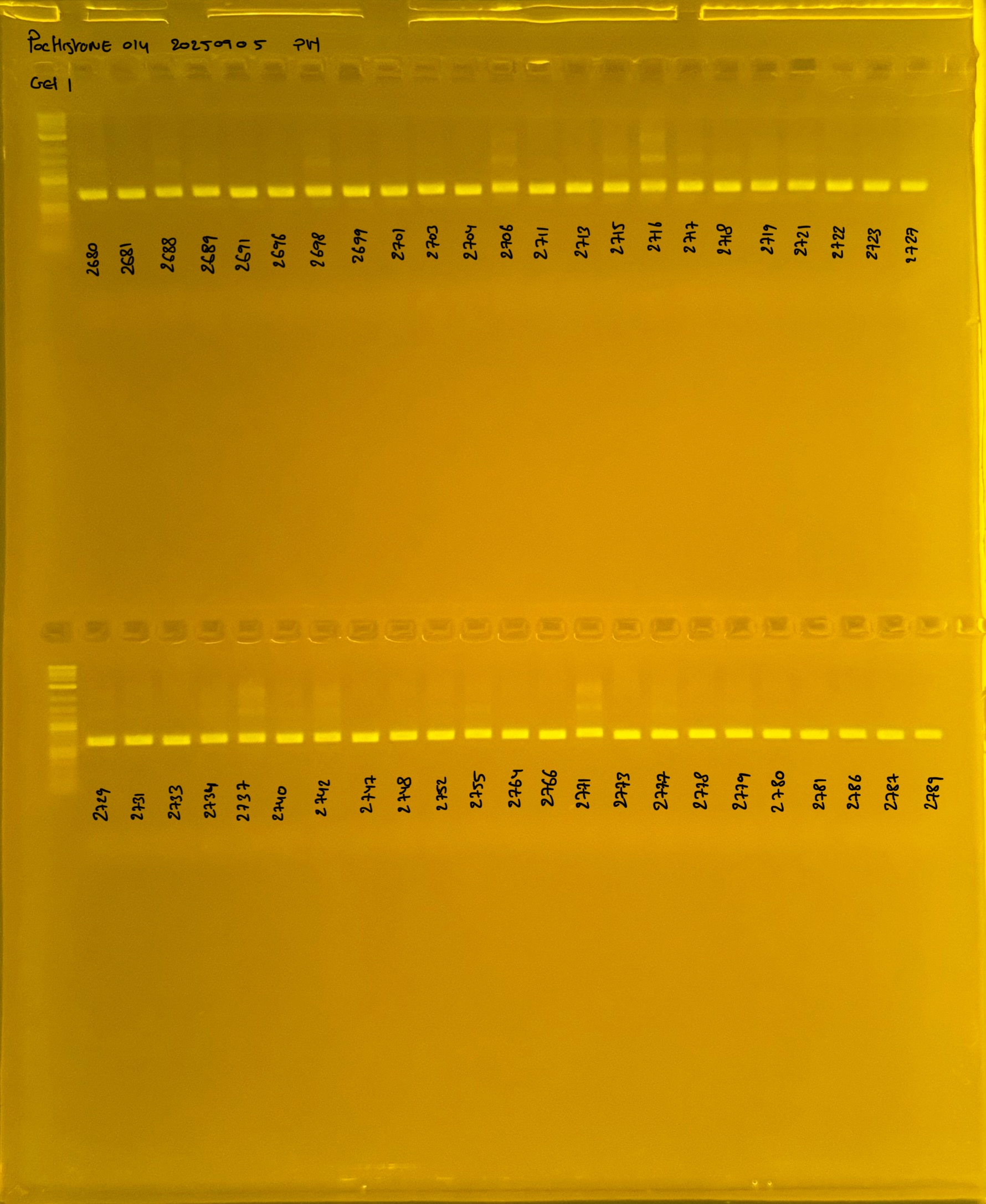
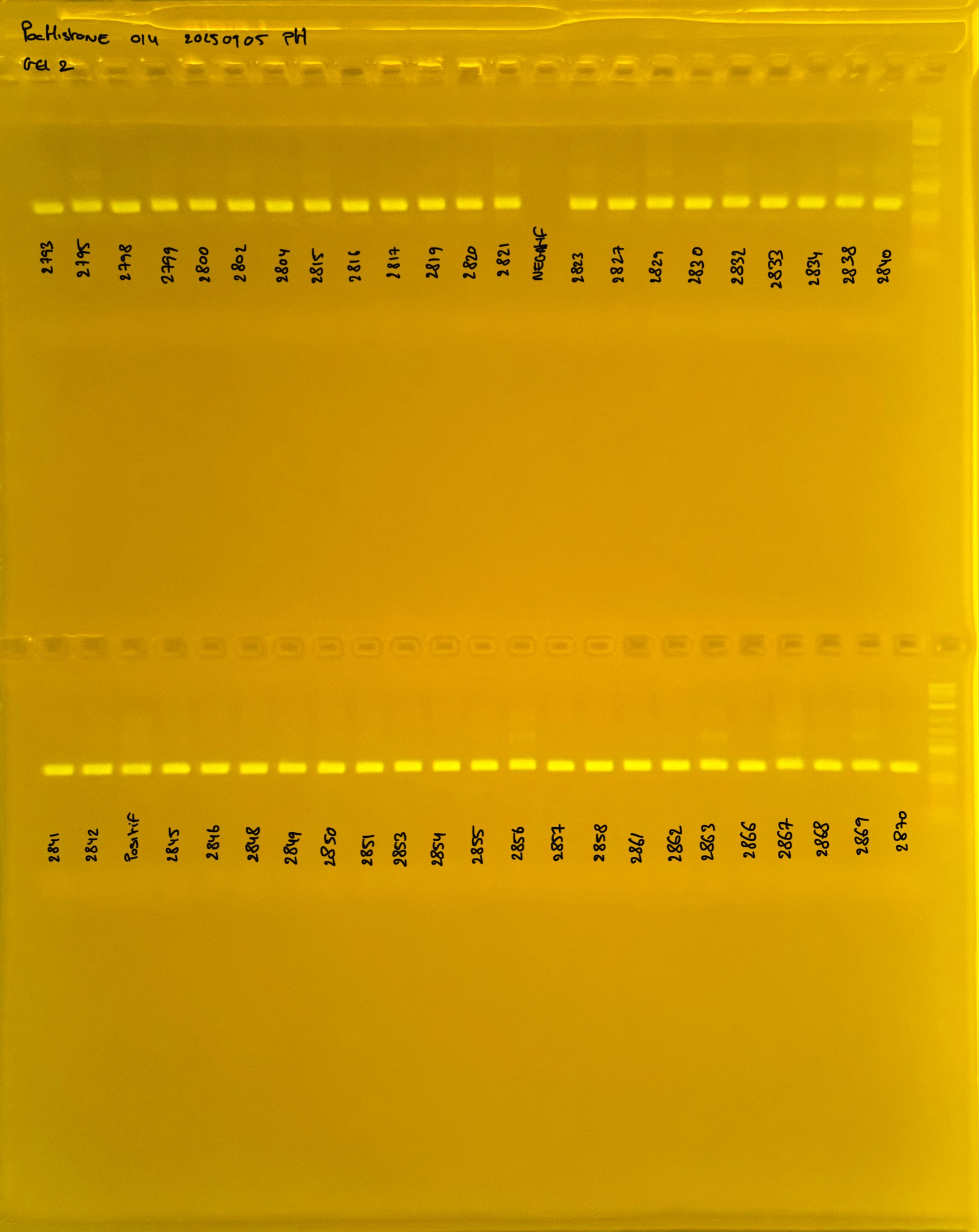
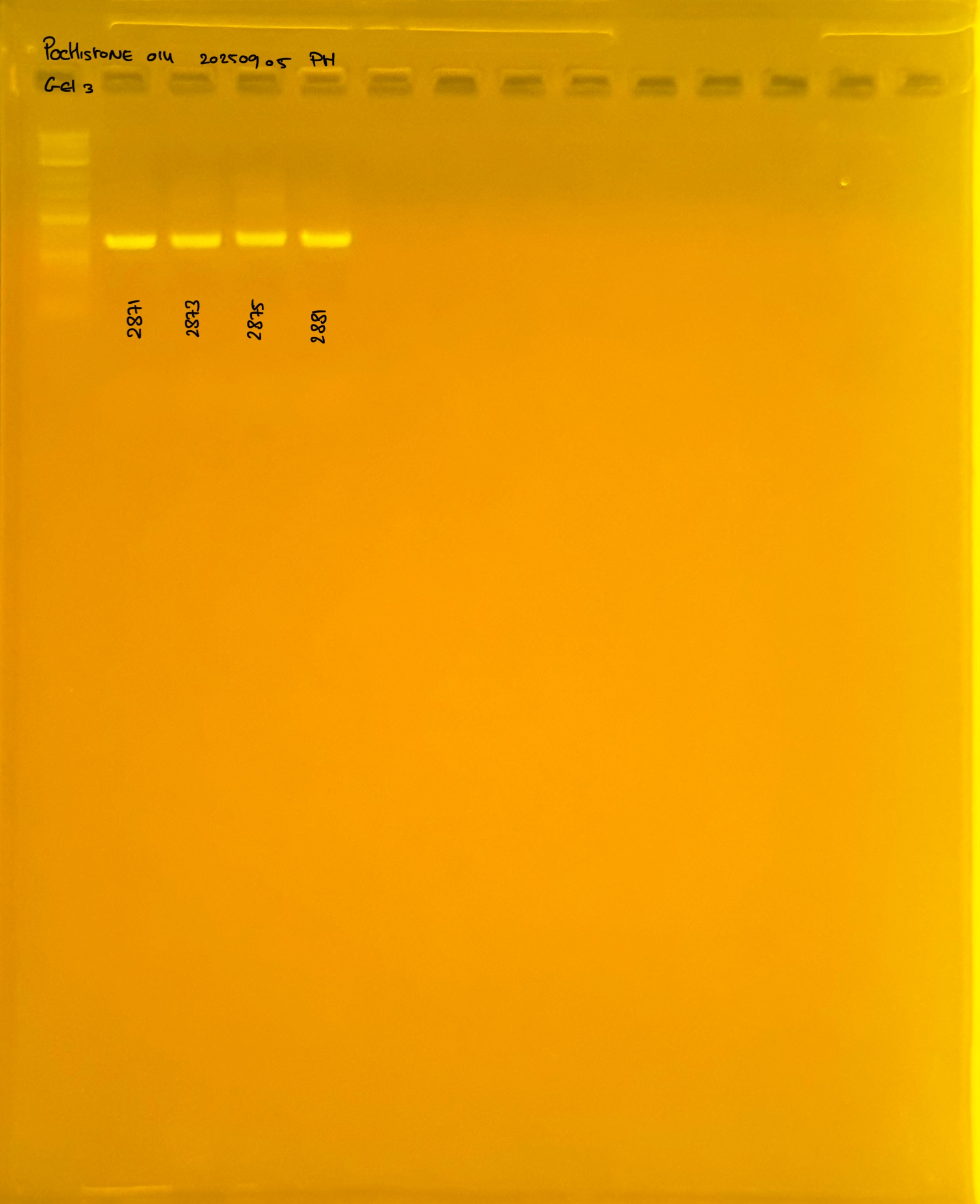
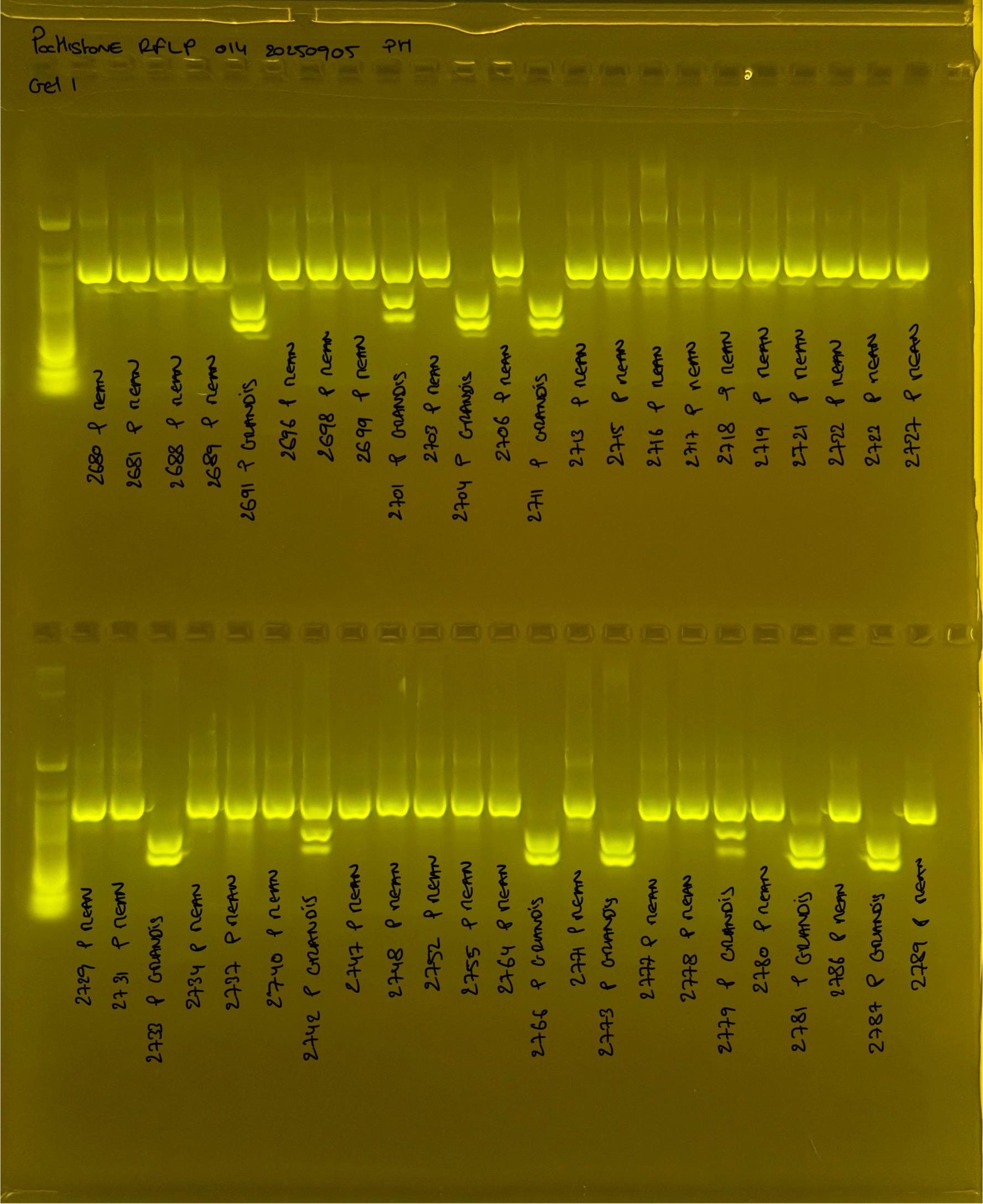
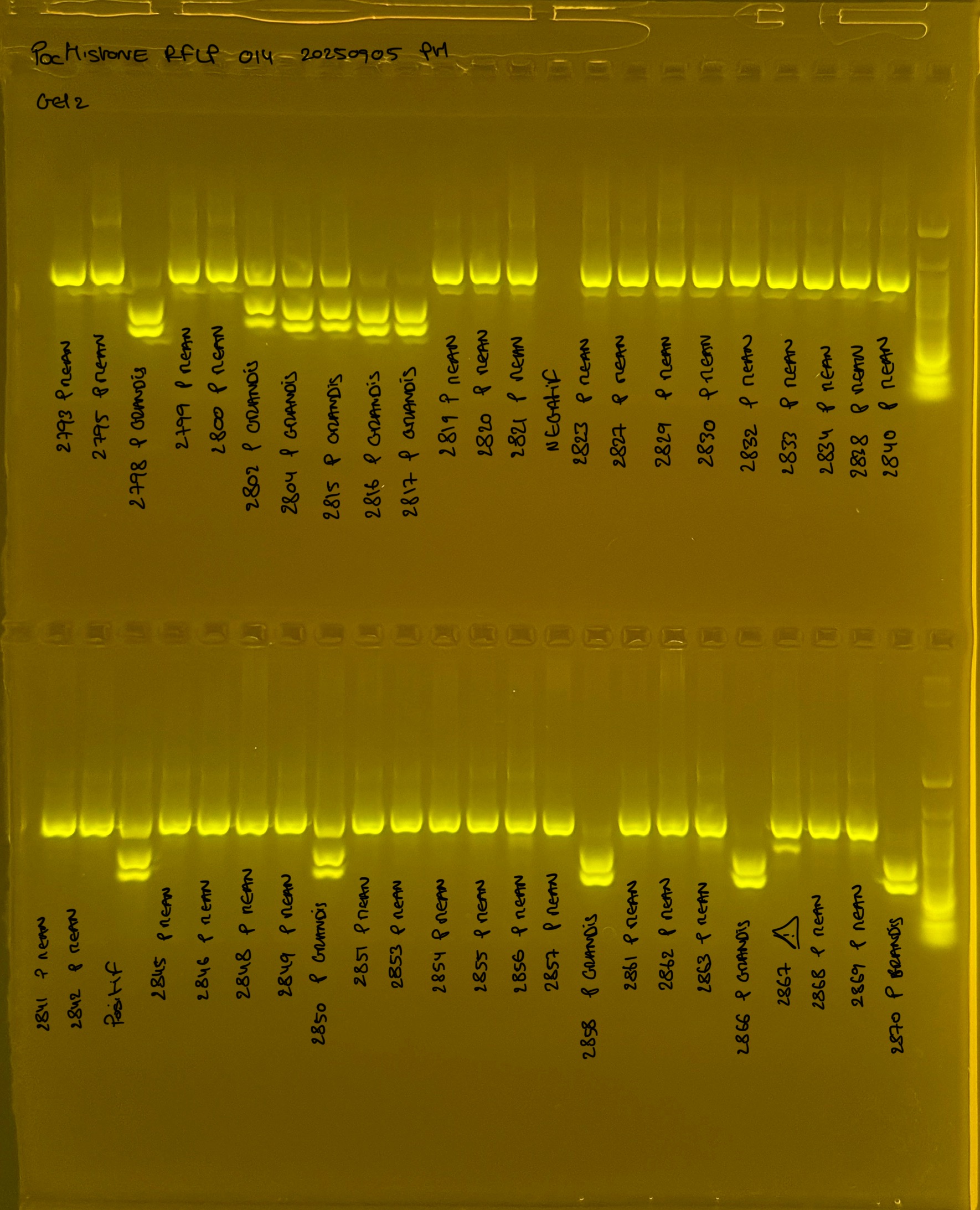
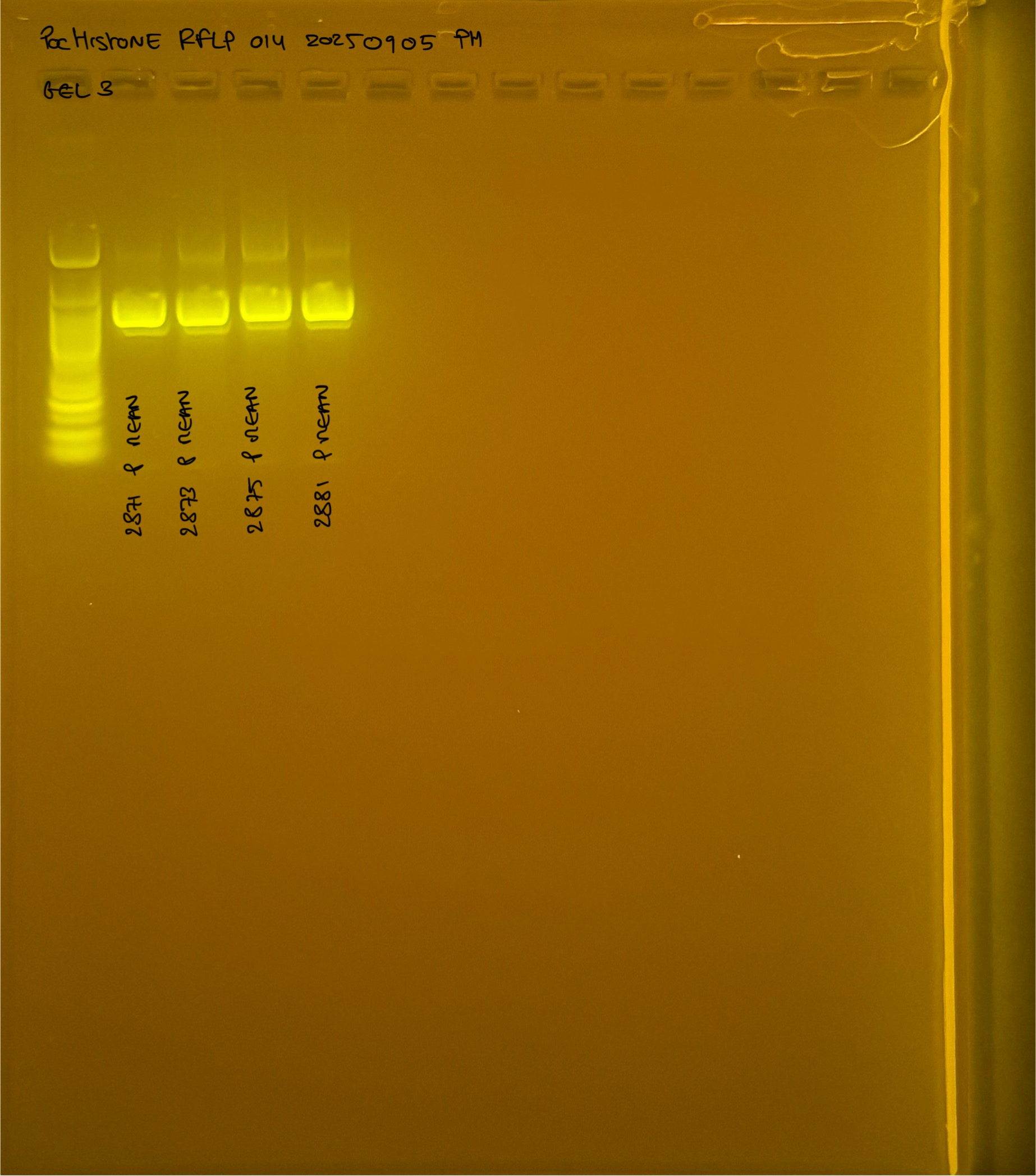
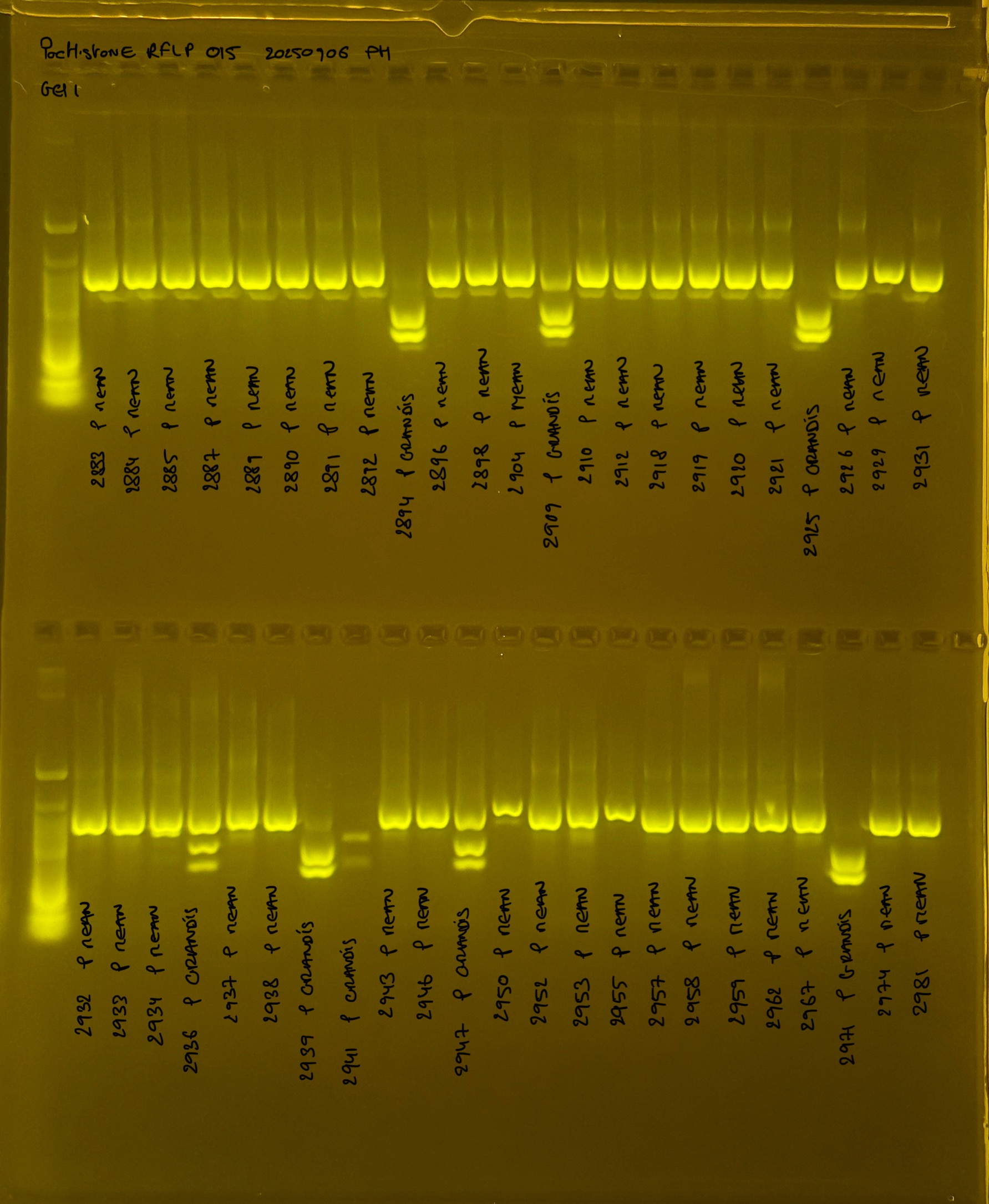
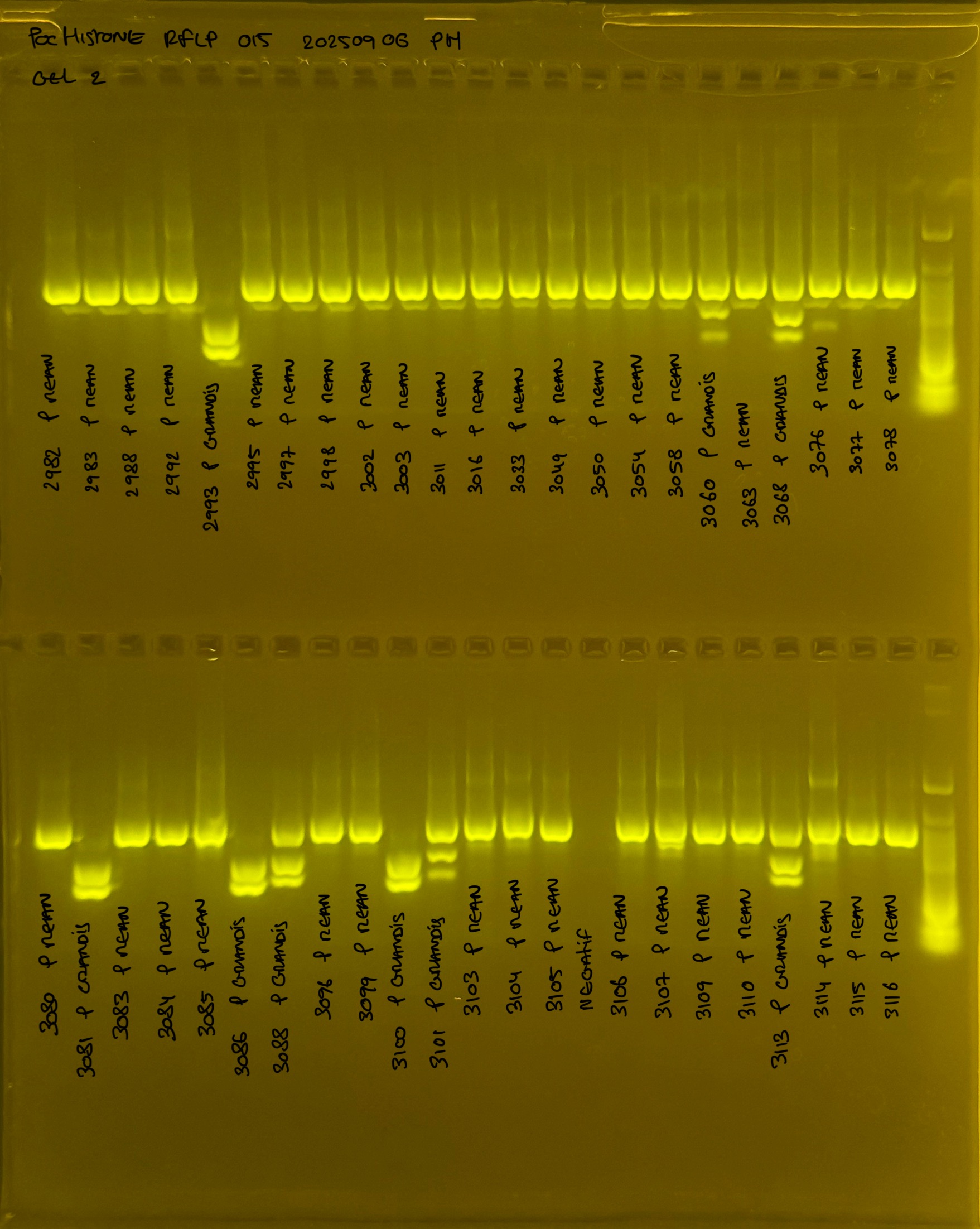
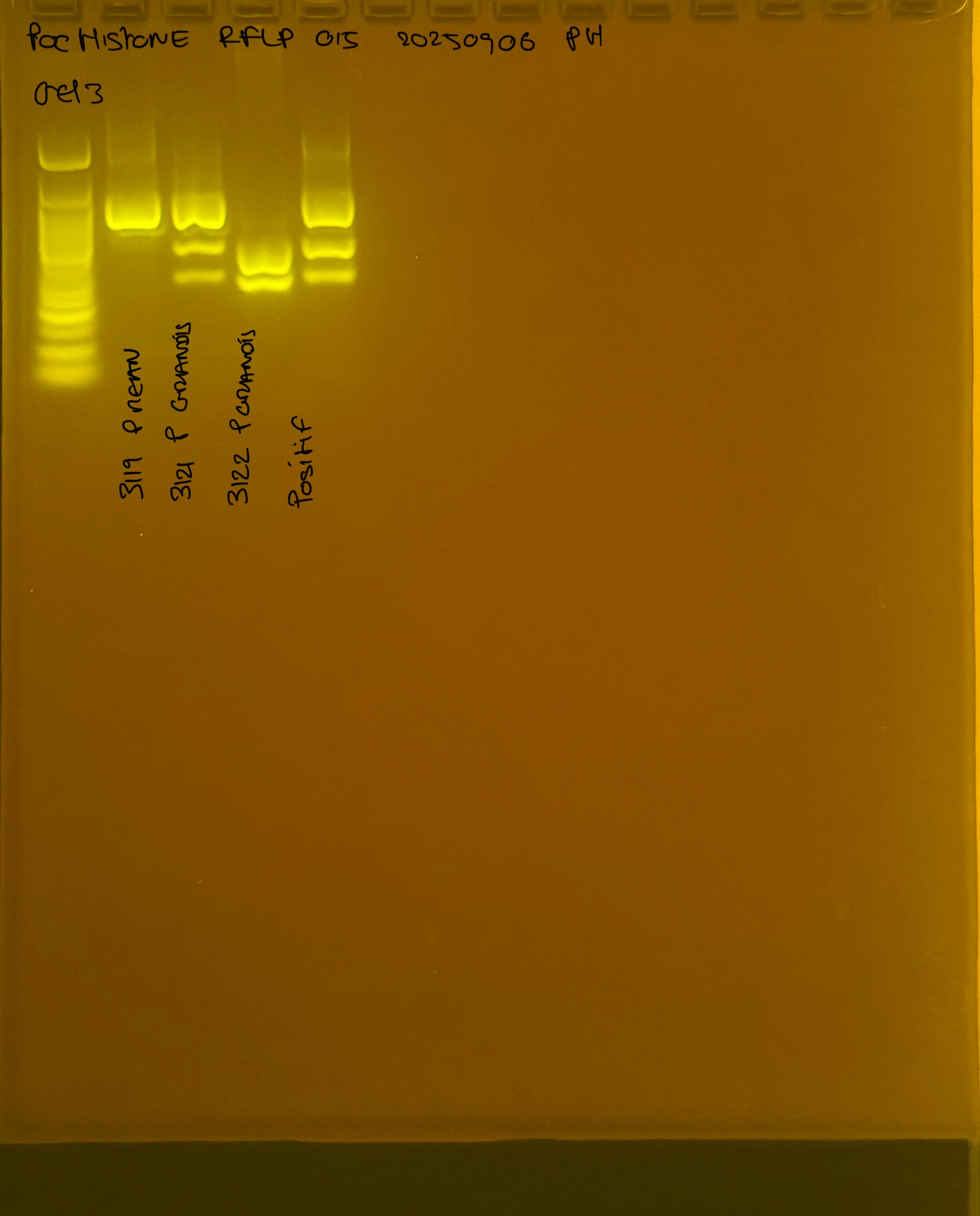
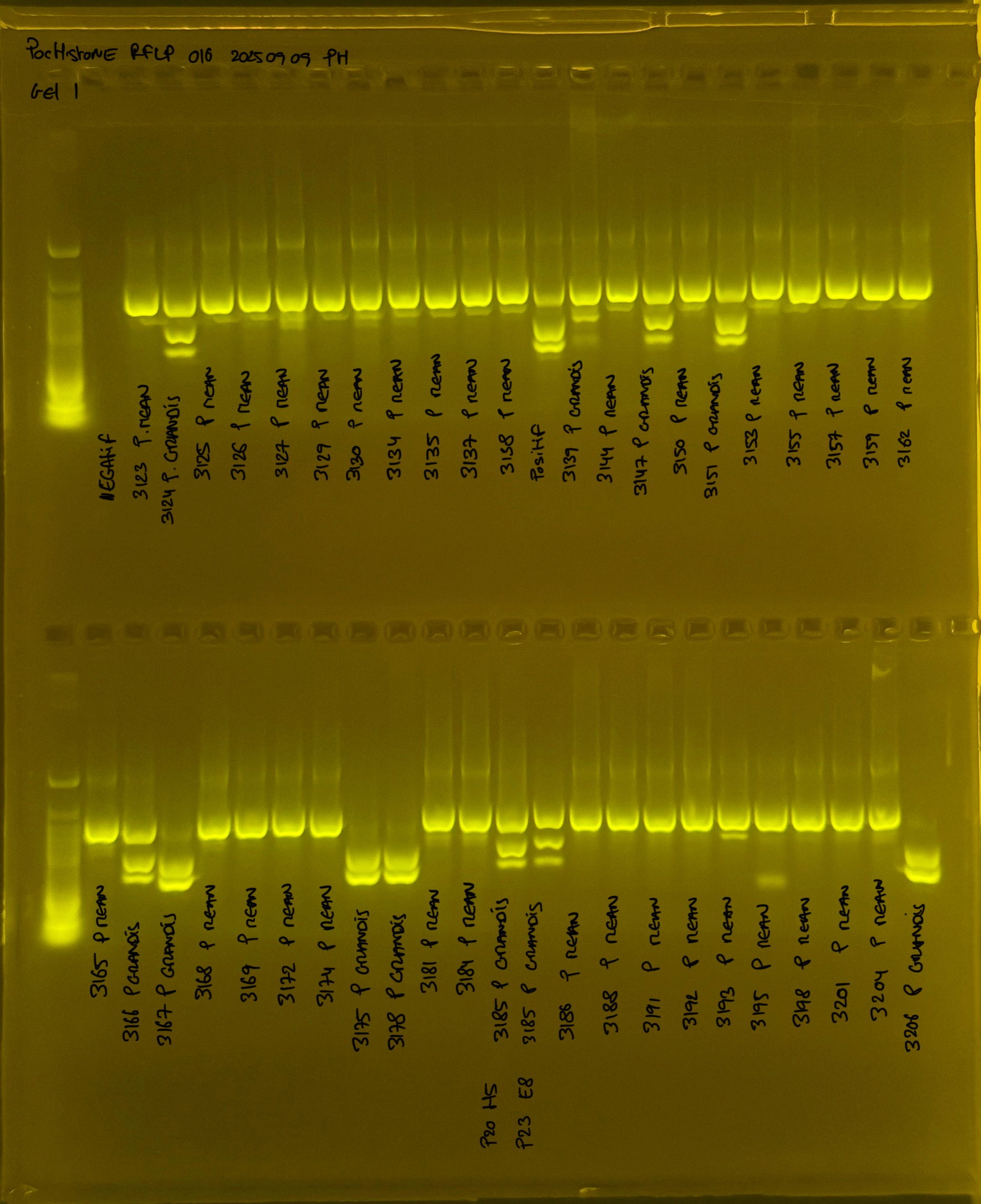
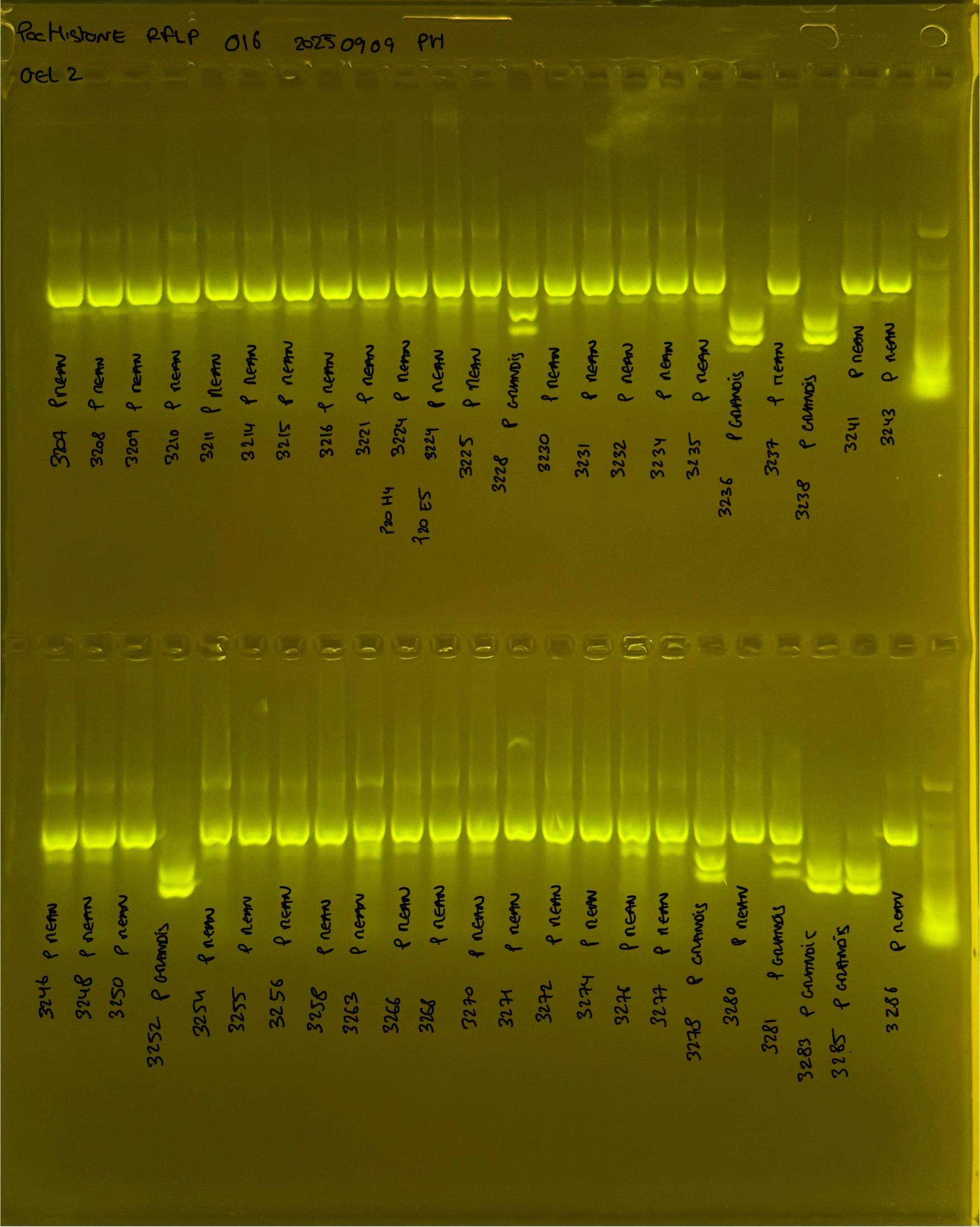
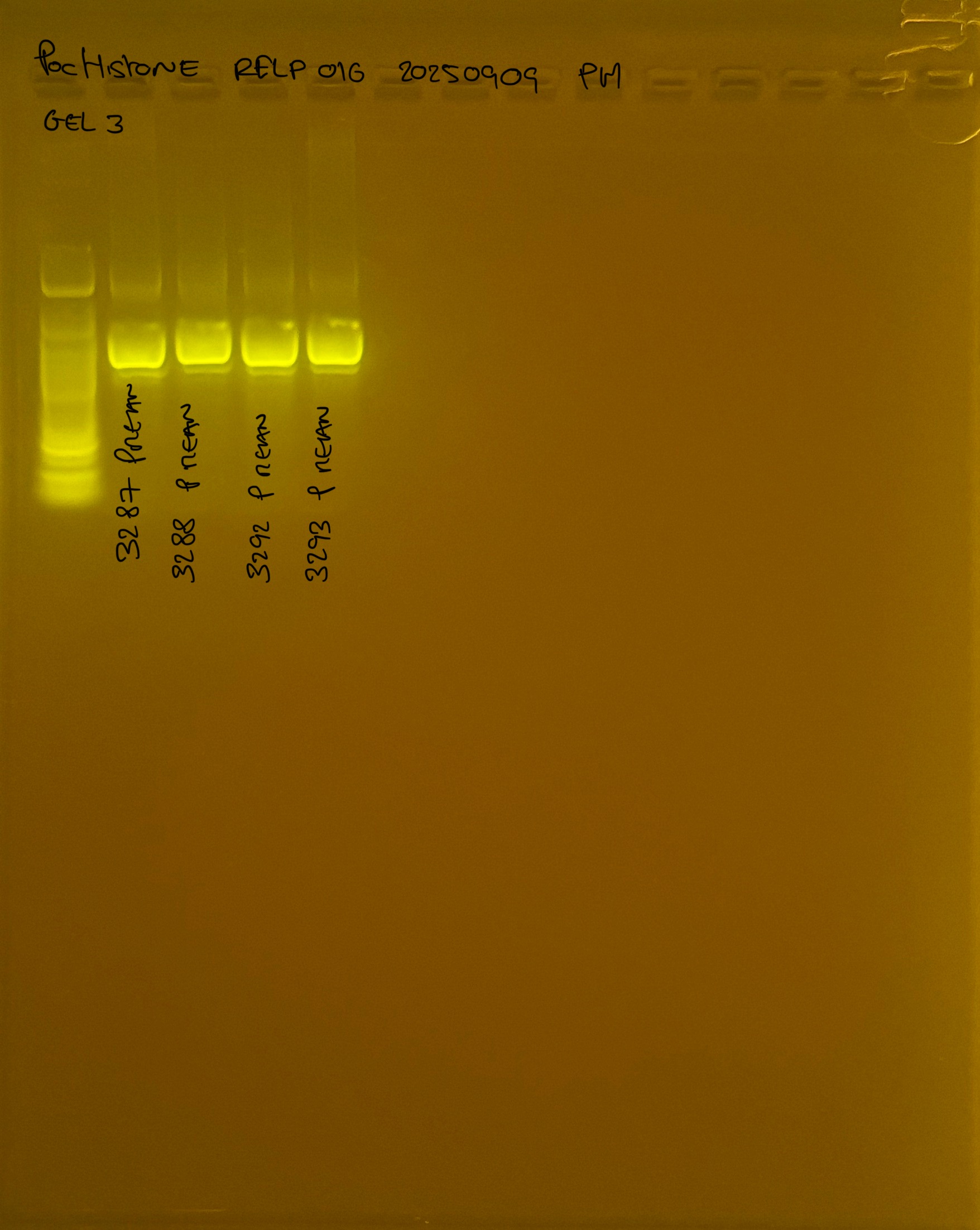
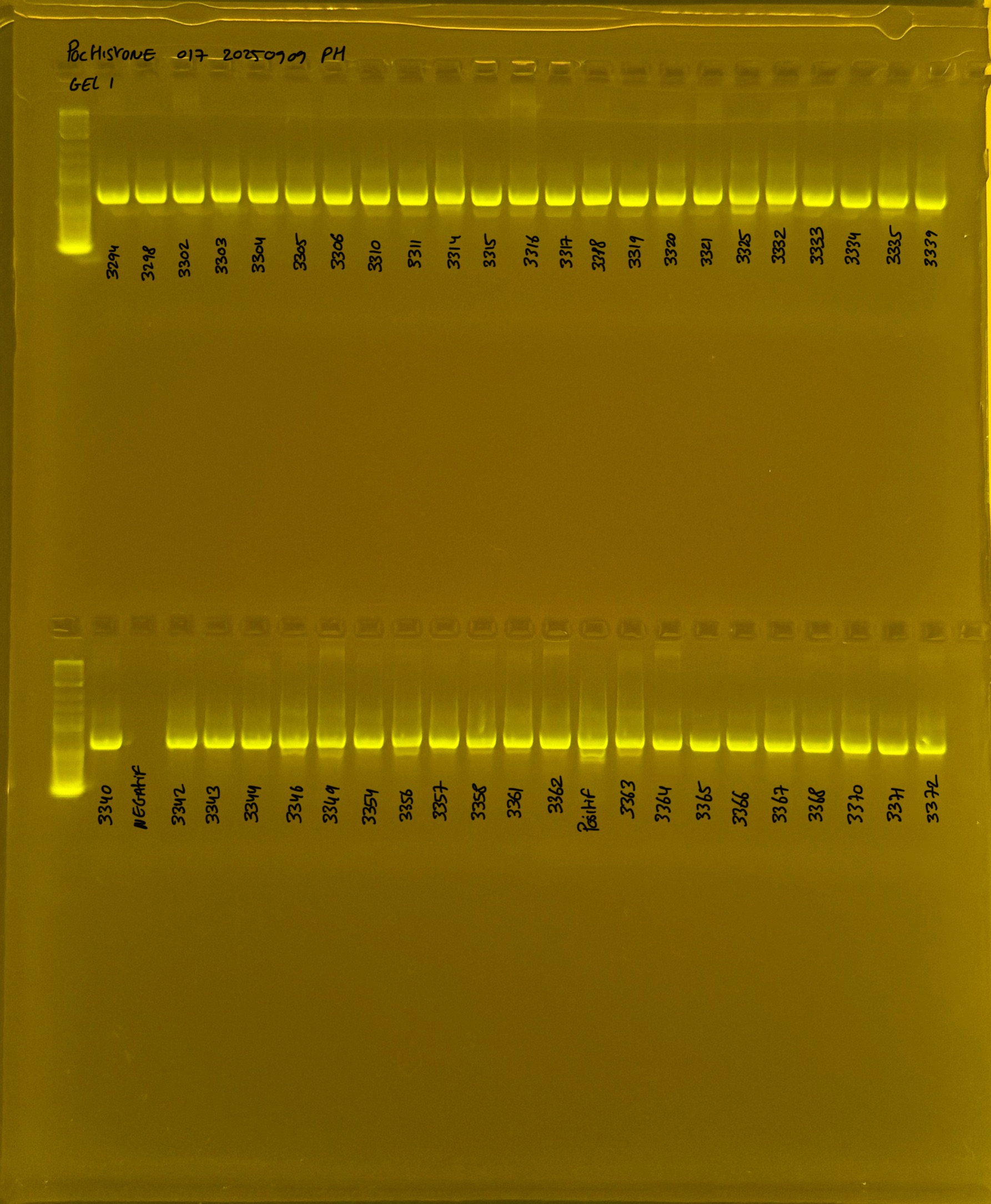
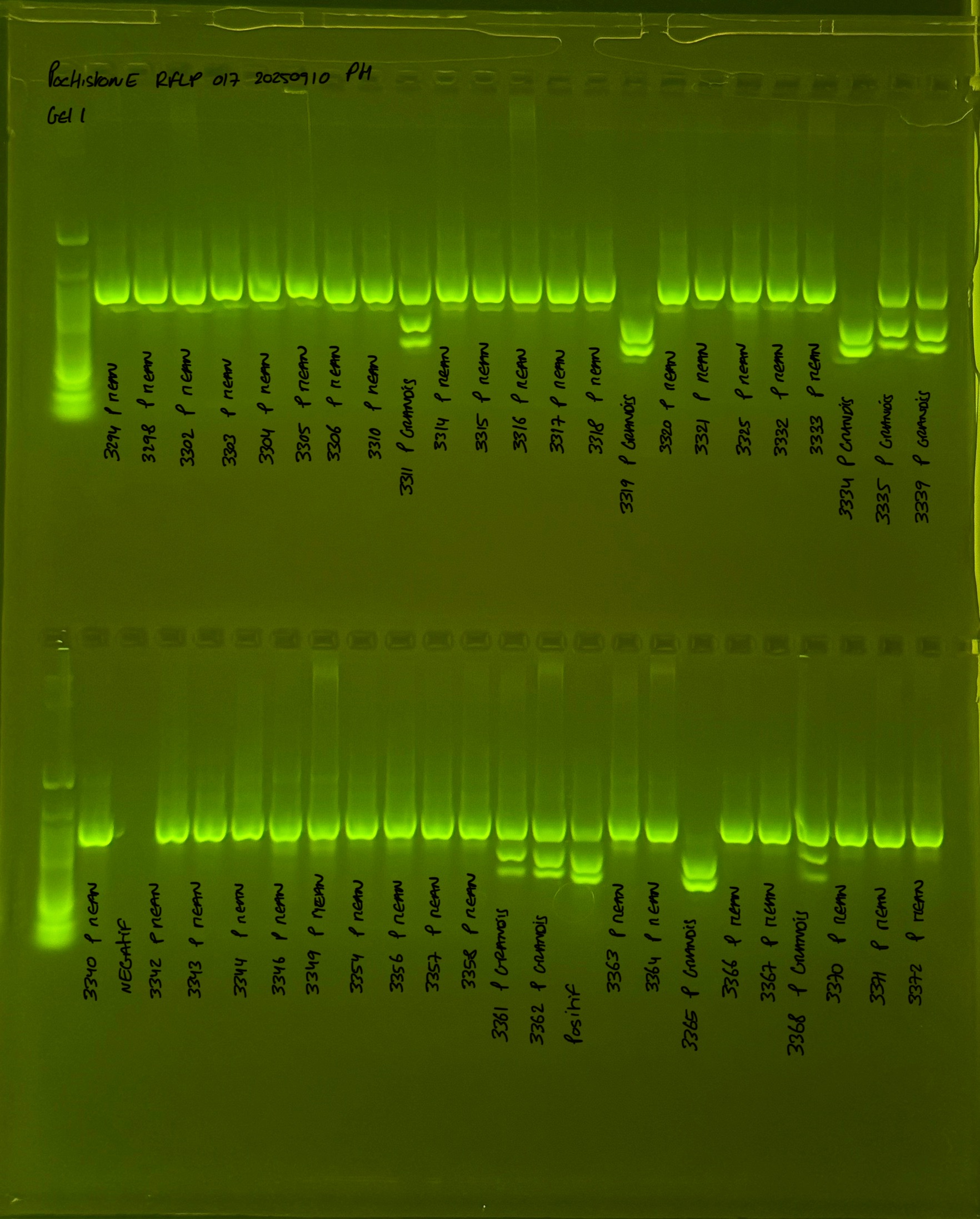
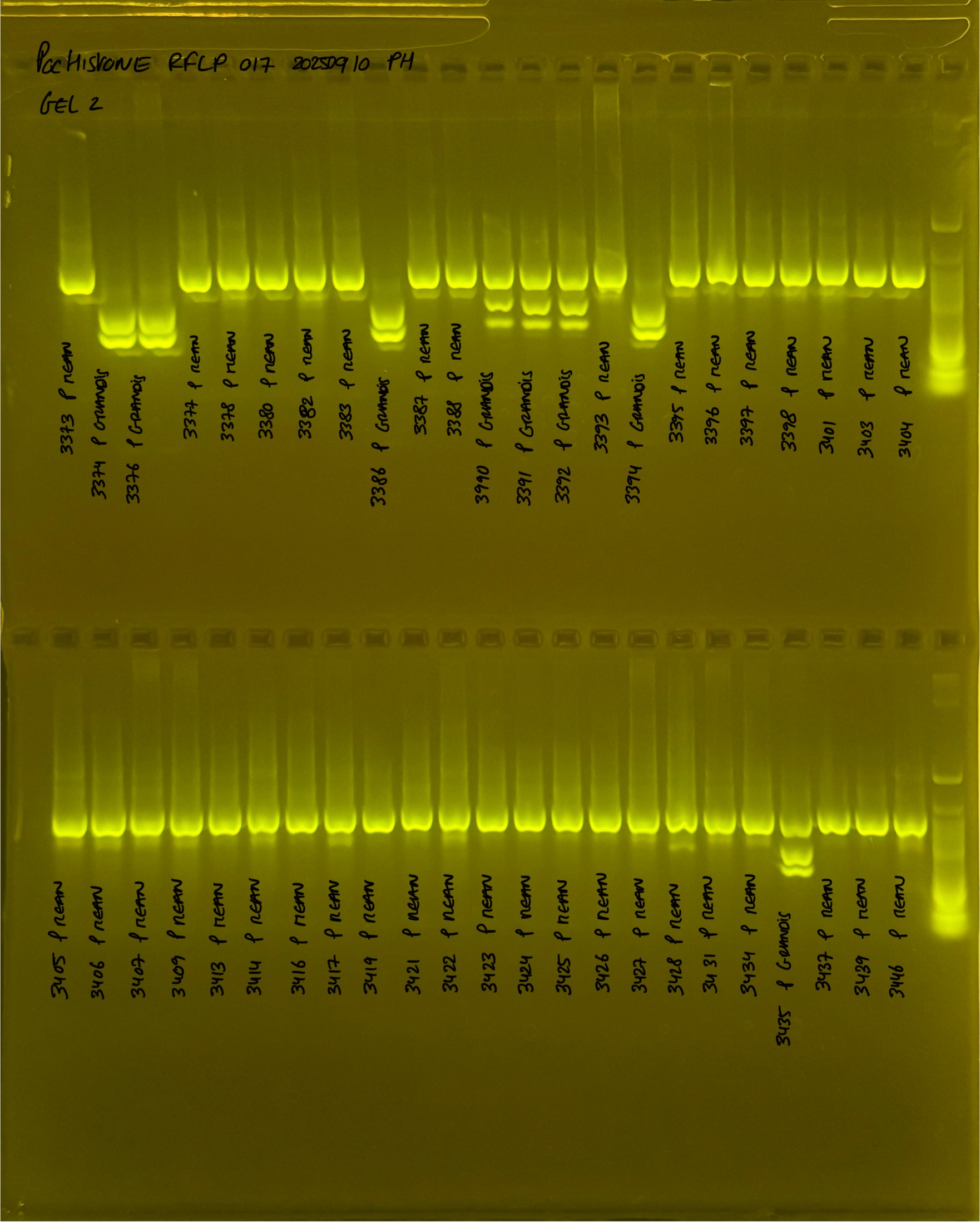
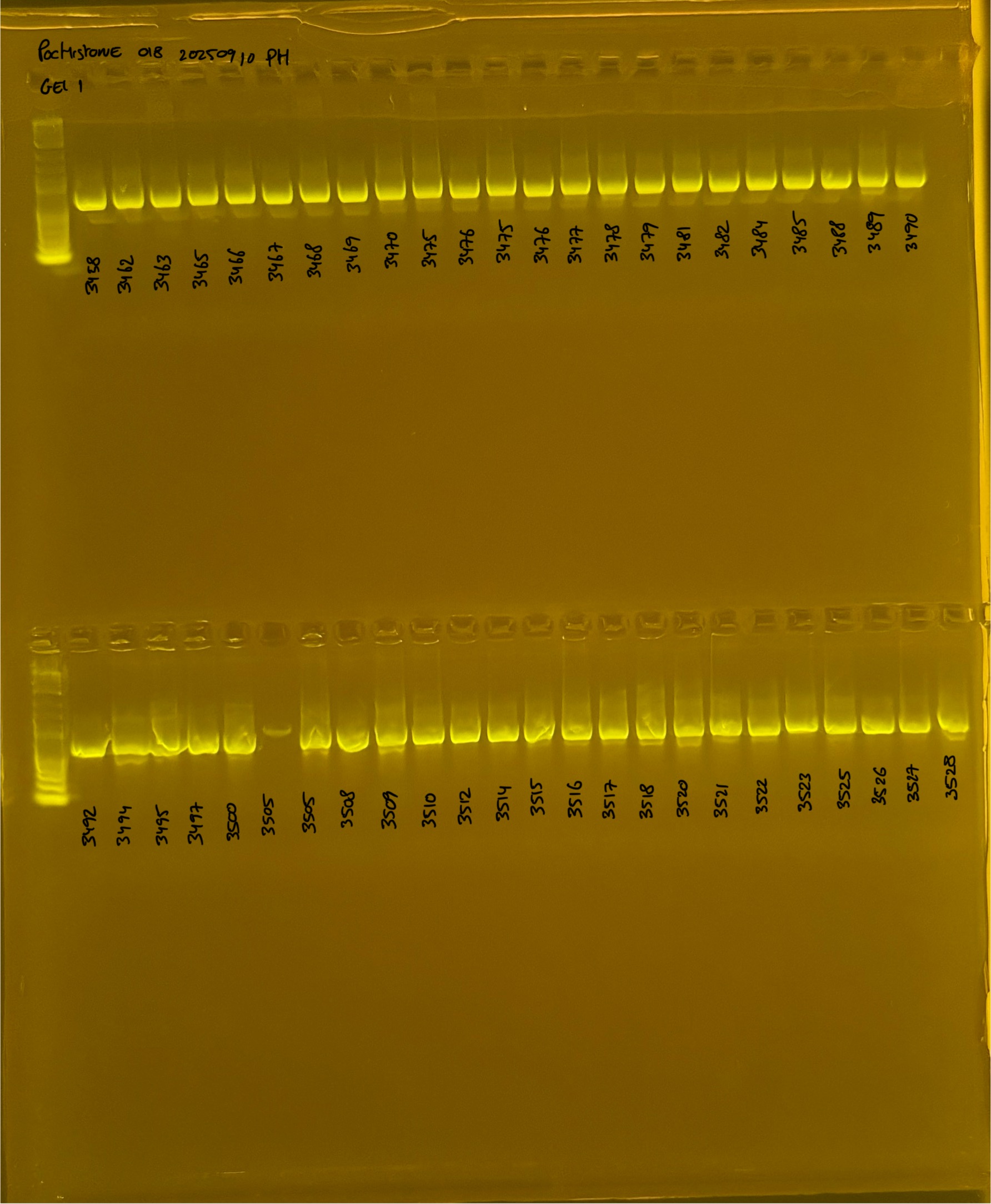
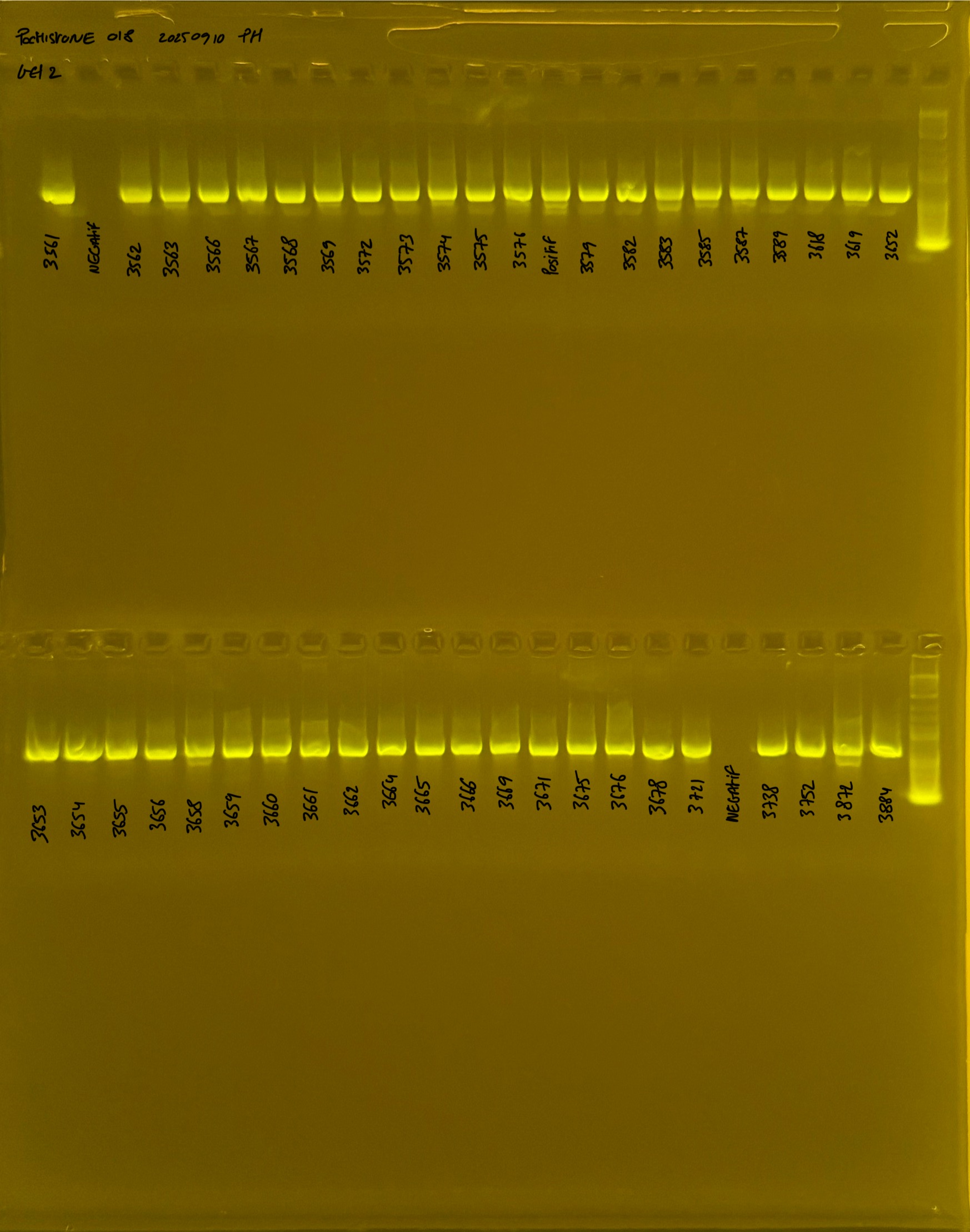
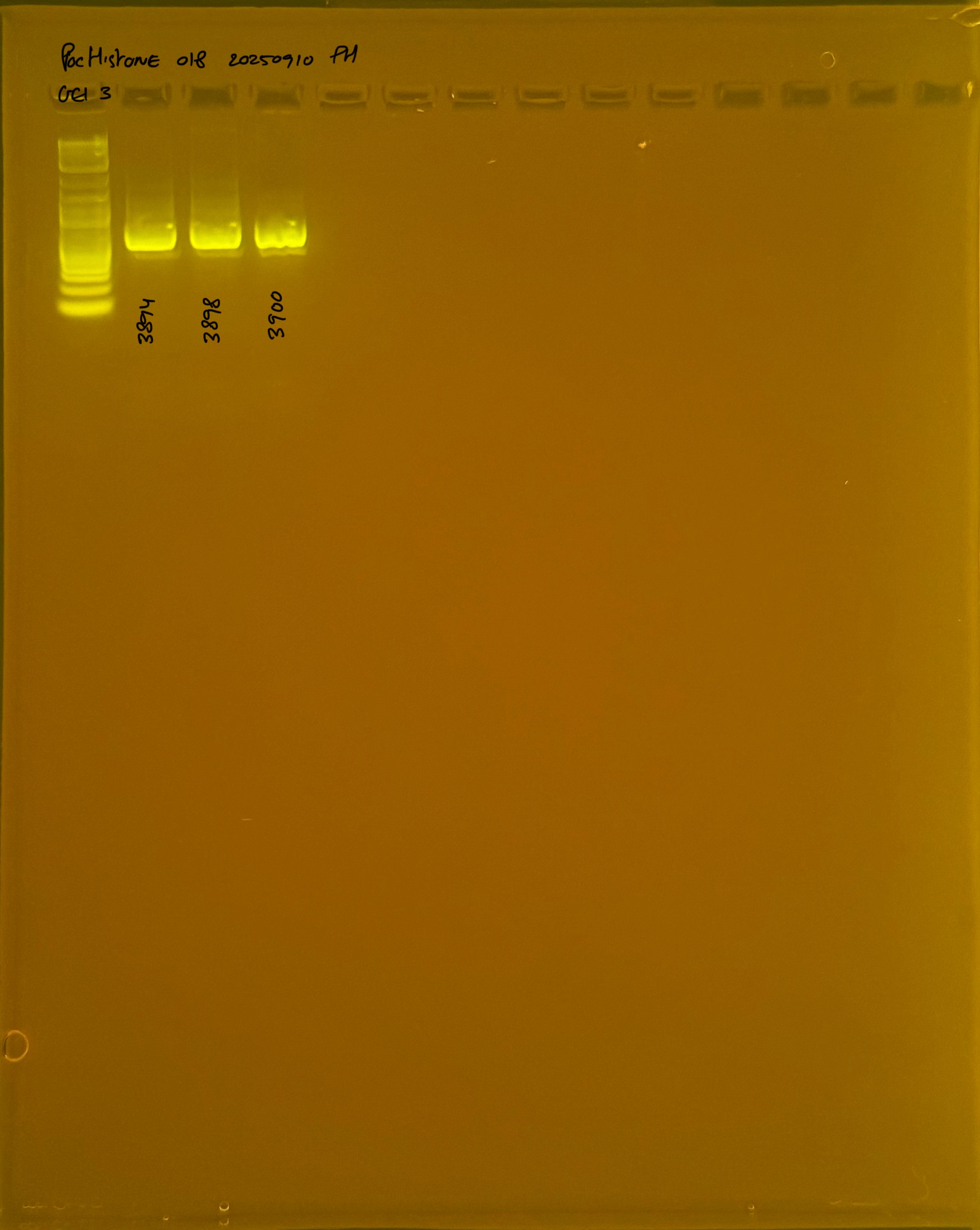
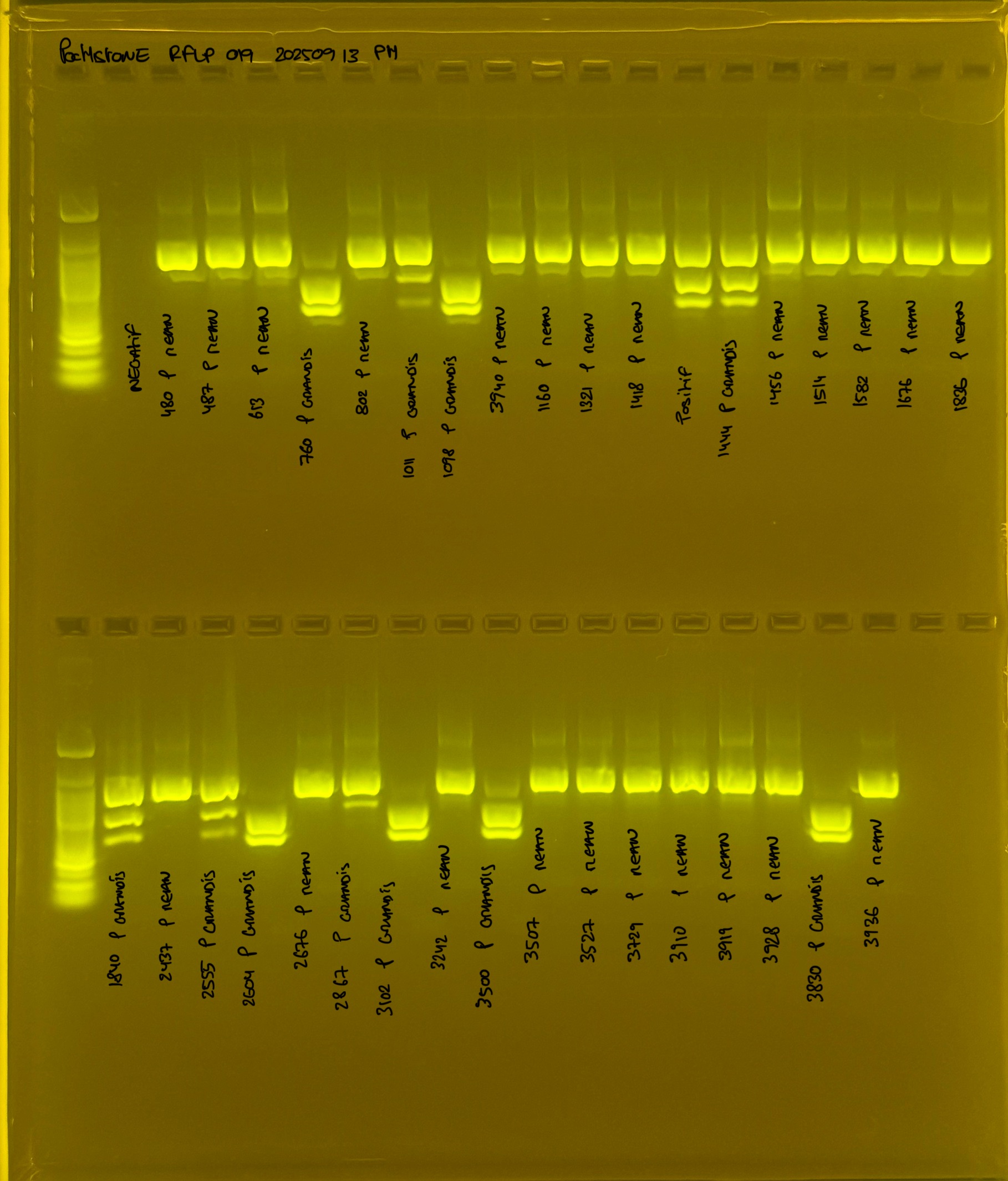
PocHistone and RFLP for species identify as Haplotype 1a (P. grandis - P. meandrina):
Protocol:
The protocol used comes from Hollie Putnam’s LabNotebook and is available here