Pocillopora larvae competency experiment
Resume of project
Coral spawning is a phenomenon known worldwide, affecting many coral species. However, certain aspects of this reproduction are not well understood, particularly the recruitment phase in species such as Pocillopora spp. Many studies have been conducted on Pocillopora acuta, but very few have been done on P. meandrina, P. verrucosa, P. tuahineensis, P. grandis and P. cf. effusa. Crucial for reef development, genetic mixing, habitat creation, and increasing reef complexity, the pre-competency and competency phases must be studied to complete the reproductive cycle of Pocillopora spp. In French Polynesia, Harnay et al. 2024 documented the reproduction of P. meandrina, P. verrucosa, P. tuahineensis, and P. cf. effusa for the first time, providing more insights and possibilities for increasing research on the settlement phases.
In this context, this study aims to define a period of pre-competency and competency based on the protocol of Randal et al. 2024 for P. meandrina, P. verrucosa, P. tuahineensis, P. cf. effusa and P. grandis in French Polynesia on the island of Mo’orea.
This step of understanding will help us fill in missing parts of the reproductive cycle and also provide more knowledge about recruitment in cryptic species dominant on many coral reefs worldwide, such as on the island of Mo’orea.
Protocol
In the context of this experiment, we based our work on the research conducted by other teams on different species. Our protocol will therefore be correlated with Randal et al. 2024.
Global plan
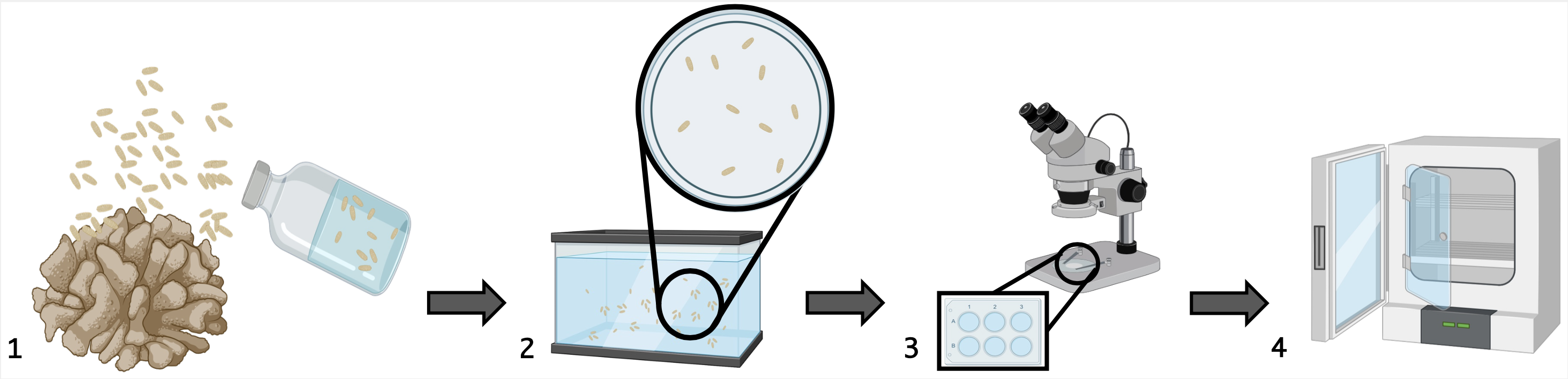 .
.
Fig 1: 1) Collection of larvae directly from the lagoon of Mo’orea using an empty bottle (creation of a siphon next to Colony 2) Maintenance and growth of coral larvae in an aquarium kept in an open circuit. 4) Counting of larvae under a microscope with the help of a blue fluorescent lamp to locate the larvae. 5) Placement of the larvae into the 6-well plates designated for them (n=6 conditions, n=10 larvae per well).
Coral spawning monitoring
The collection and detection of spawning for each species will be based on the study by Harnay et al. 2024. The colonies will not be collected and will remain in the lagoon. A plastic bottle (rinsed with seawater 3 times) will be used to collect the coral larvae. (step 1 on the cartoon).
Coral larvae maintenance
Once back at the GUMP station, the bottles containing larvae from different genotypes are mixed and transferred to an aquarium supplied with an open circuit. Water changes will occur every 2 days. To avoid losing the larvae and to minimize their stress, the larvae will be collected using a 120-micron filter and stored in a separate aquarium.
Crustose Coralline Algae
For this experiment, three different species of CCA (crustose coralline algae) will be used:
- Porolithon cf. onkodes
- Neogoniolithon cf. megalocystum
- Lithophyllum sp.
The species listed above were selected based on the paper by Vizon et al. 2024. The three species are present in the lagoon (back reef) of Mo’orea and will be collected one day before the coral spawning date (according to the lunar calendar). They will be stored in an aquarium and placed on supports to minimize contamination by other algae or organisms not related to these species. For the benefit of the experiment, new CCA will be collected (from the same sites) every week to ensure the highest possible quality and to maintain the microbiome. Additionally, to avoid compromising species identification, all samples will be sequenced and analyzed.
Coral ruble
In order to increase recruitment supports, we will also use rubble found at the sampling site (CCA, larvae, rubble). To avoid introducing bias into the experiment, the rubble will come from the same dead coral colony, devoid of CCA. The dead coral skeletons collected will come from old colonies of Pocillopora spp. They will also be collected 1 day before the spawning dates and stored in an aquarium and placed on supports to minimize contamination by other algae or organisms. For the benefit of the experiment, a new colony will be collected (from the same sites) every week to ensure the highest possible quality and to maintain the microbiome.
Design experimental
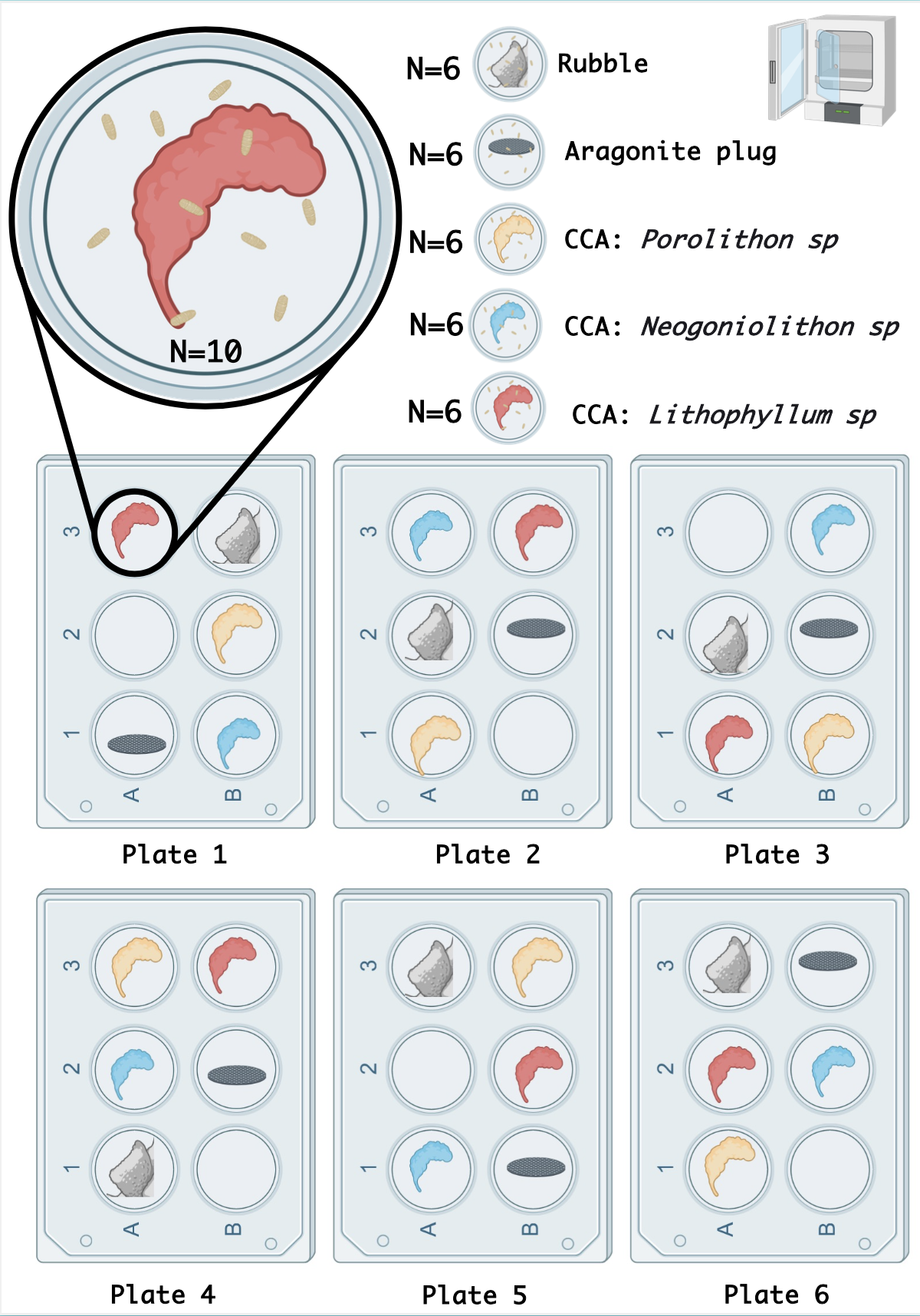
Every morning after spawning monitoring, all the plates will be examined under a microscope equipped with a blue fluorescent light to detect any larvae beginning the competency phase. These will be recorded and entered into the Excel table available here. After examination, the larvae and CCA fragments will be placed in 1 ml tubes filled with DNA-RNA Shield and stored at -40˚C before being sent for RNA and/or microbiome analysis.
After examination and sample storage, the plates will be placed in a bleach solution (20% concentration) for disinfection for 12 hours, then rinsed with DI water and dried for 12 hours.
After these steps, new 6-well plates will be used, and 10 larvae will be placed per well. For each well, a support (CCA, rubble, and plug) will be pre-cut with a Gryphon saw to ensure a similar size between replicates. The size will be 1 cm². Each support will be photographed with a ruler to later calculate the surface area. The placement of the supports will be determined randomly.
The 6-well plates will then be placed in incubators maintained at 26˚C and monitored with temperature probes. An AquaIllumination LED light will be placed in each incubator and adjusted according to the light intensity present in the natural environment.
Temperature and light data will be recorded morning and evening throughout the experiment. The placement of the 6-well plates will also be changed randomly twice a day (4 and 8 hours after exposure to light) to avoid creating light intensity bias.
The experiment will continue until recruitment is observed.
